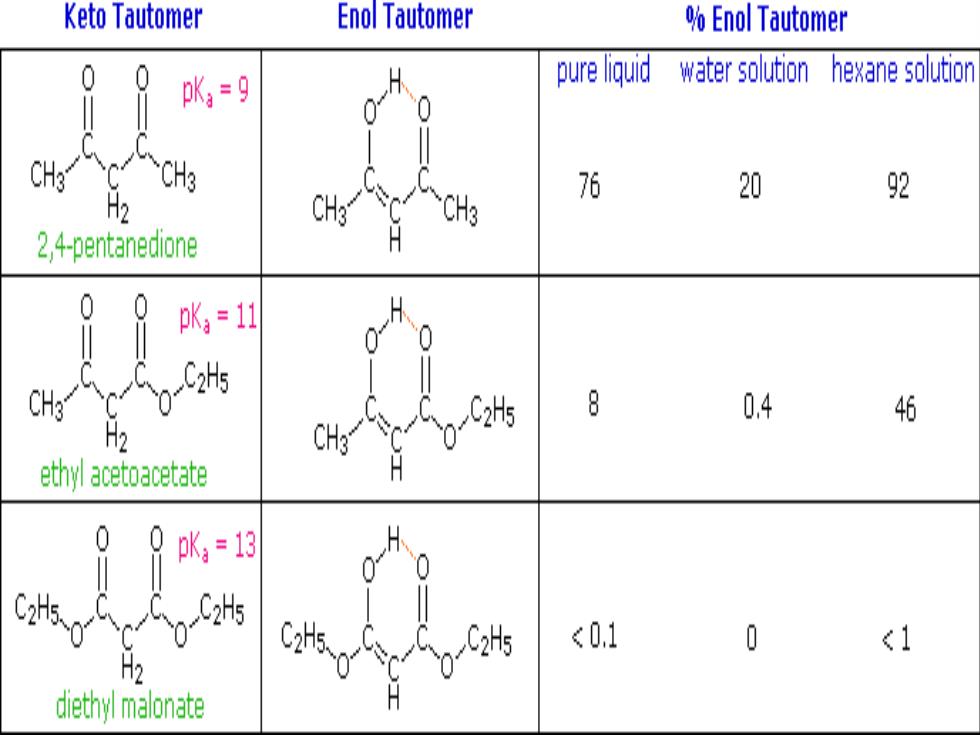
Keto Tautomer Enol Tautomer %Enol Tautomer 0 pKa=9 pure liquid water solution hexane solution CH3 CH3 76 20 92 H2 2,4-pentanedione 0 pK,=11 CH3 8 0.4 46 H2 ethyl acetoacetate 0K=13 C2H5 <0.1 0 <1 diethy malonate

Sec 2 Preparation Functional group transformations .Aldehydes can be synthesized by the oxidation of primary alcohols,or by the reduction of esters,acid chlorides,or nitriles. Ketones can be synthesized by the oxidation of secondary alcohols. Carbon-carbon bond formation C-C Bond cleavage
Sec 2 Preparation ◼Functional group transformations ⚫Aldehydes can be synthesized by the oxidation of primary alcohols, or by the reduction of esters, acid chlorides, or nitriles. ⚫Ketones can be synthesized by the oxidation of secondary alcohols. ◼Carbon-carbon bond formation ◼C-C Bond cleavage

1.Functional group transformations Aldehydes can be synthesized by the oxidation of primary alcohols ,or by the reduction of esters,acid chlorides,or nitriles. KCN 1.DIBAH,toluene R一X R一C三N 2.H300 Alkyl halide Nitrile 二异丁基氢化铝 Fig.1.Synthesis of an aldehyde from an alkyl halide with 1C chain extension
1. Functional group transformations ◼Aldehydes can be synthesized by the oxidation of primary alcohols ,or by the reduction of esters, acid chlorides, or nitriles. 二异丁基氢化铝
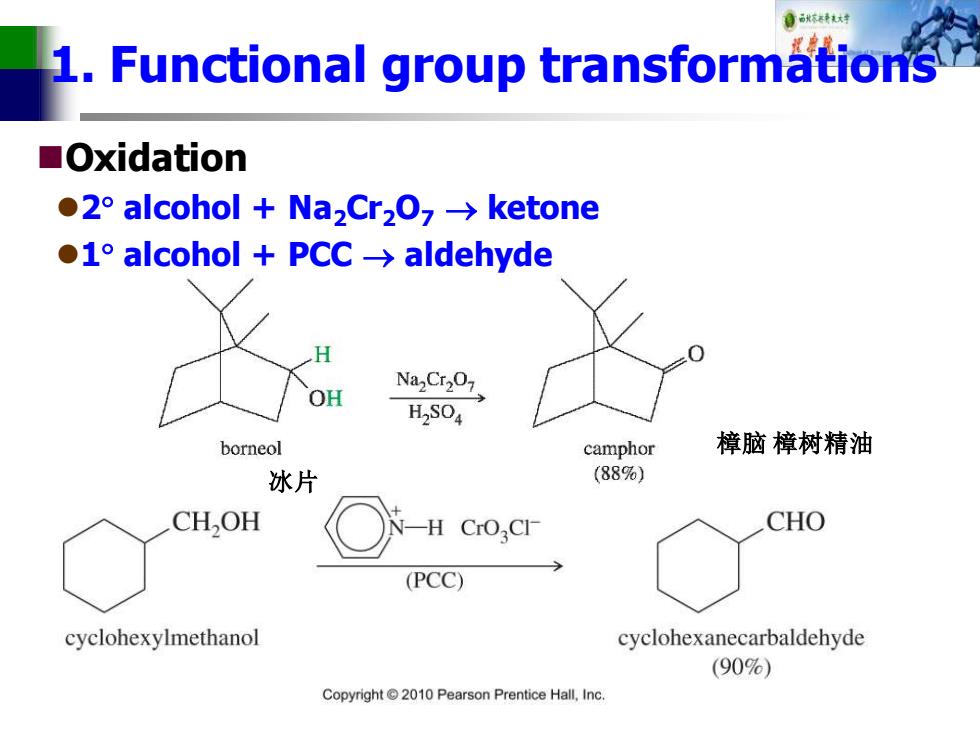
自秋不转试对 1.Functional group transformations ■Oxidation ●2°alcohol+Na2Crz0,→ketone ●I°alcohol+PCc→aldehyde NazCr207 OH H,SO4 borneol camphor 樟脑樟树精油 冰片 (88%) CH,OH CrOCI CHO (PCC) cyclohexylmethanol cyclohexanecarbaldehyde (90%) Copyright2010 Pearson Prentice Hall.Inc
1. Functional group transformations ◼Oxidation ⚫2 alcohol + Na2Cr2O7 → ketone ⚫1 alcohol + PCC → aldehyde 冰片 樟脑 樟树精油
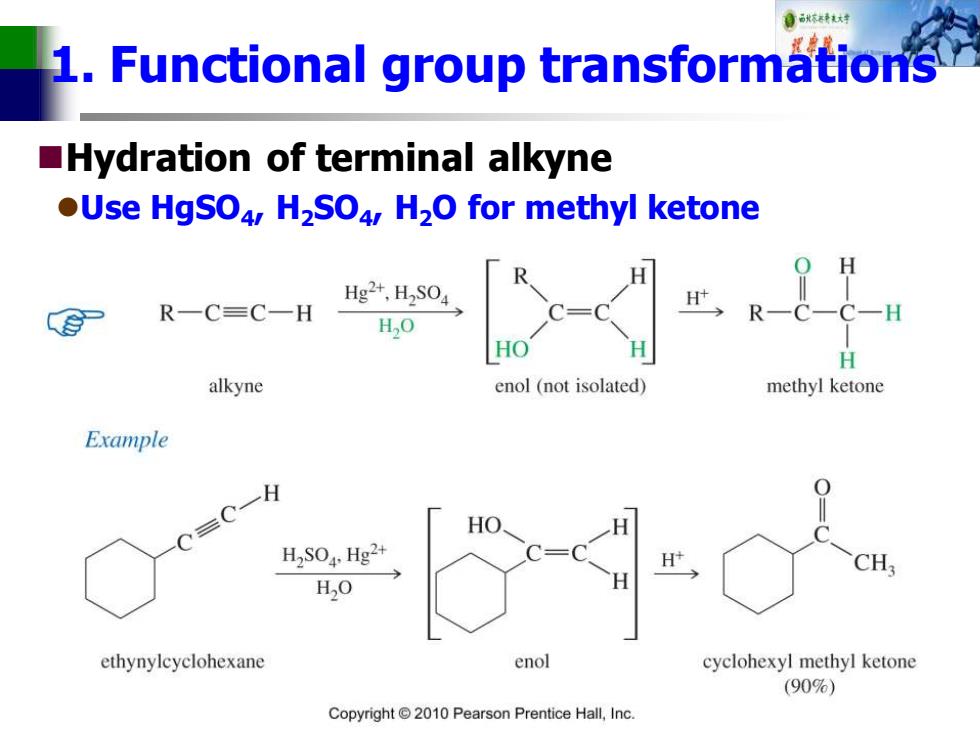
1.Functional group transformations Hydration of terminal alkyne OUse HgSO4,H2SO4,H2O for methyl ketone Hg2+,H2S04 R一C=C一H H,O HO H alkyne enol (not isolated) methyl ketone Example C=C一 H H2S04,Hg2+ H CH: H,O ethynylcyclohexane enol cyclohexyl methyl ketone (90%) Copyright 2010 Pearson Prentice Hall,Inc
1. Functional group transformations ◼Hydration of terminal alkyne ⚫Use HgSO4 , H2SO4 , H2O for methyl ketone