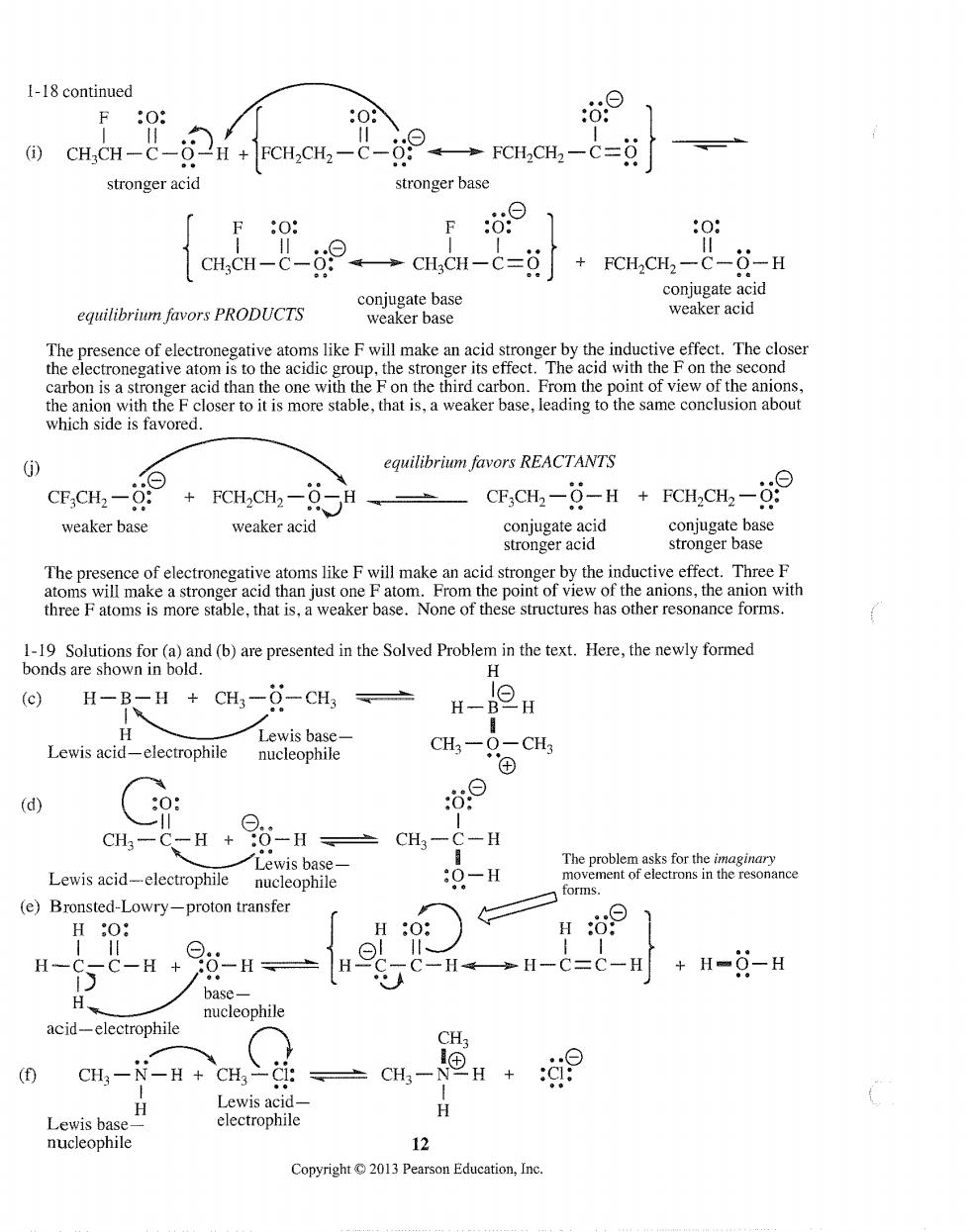
1-18 continued F:O: 08 :69 CH;CH-C- 2 H+FCH2CH2- →FCH,CH2-C=0i stronger acid stronger base F:O: F ⊙ cuen-2- 9。→cH,CH-c=9j FCHCH2-C-0-H equilibrium favors PRODUCTS tive ator s like f will make an acid st by the inductive effect.The closer m的 carbon is a equilibrium favors REACTANTS CF.CH.- FCH2CH2-6 G,CH-点-H+CHcH,-9 weaker base weaker acid coniugate acid conjugate base stronger acid stronger base The pre oms e anio with three atoms is more stable that is.weaker base.None of these stuctures has other resonance forms. 1-19 Solutions for(a)and(b)are presented in the Solved Problem in the text.Here,the newly formed bonds are shown in bold. H (c) H-B-H CH3-0-CH3 H-BOH H Lewis base- Lewis acid-electrophile nucleophile CH3-O-CH3 (d) 0 CH:-C-H +:0-H CH-( -H Lewis acid-electrophile nucleophile :O-H 、foms. (e)Bronsted-Lowry-proton transfer H0: H- -H+98-H H C--C=C-+H-0-H A acid-electrophile nucleophile CH; -N-H+CH3-C1: CH3 H+ 这9 Lewis acid- H Lewis has electrophile nucleophile 12 Copyright13 Pearson Education,Inc

1-19 continued (g)CH3-G=CH2 Lewis bas nucleophile Lewis acid- electrophile 1-20 w{g8- 9一-莒= (c)The last resonance form of SO,has no equivalent form in O;Sulfur,a third row element,can have more 1-21 (a)CARBON!(the best element)(b)oxygen (c)phosphorus (d)chlorine 1-22 valence e- .123415678 He(2e- Be B N 0 D Br 1-23 (a)ionic only (b)covalent (H-O)and ionic (Nat OH) (c)covalent (H-Cand C-Li).but the C-Li bond is strongly polarized (d)covalent only (e)covalent (H-C and C-O)and ionic (Nat -OCH3) (f)covalent (H-C and C=O and C-O)and ionic (HCO,-Na+) (g)covalent only 1-24 斌、 、 (a) (b) C C 道年年 CANNOT EXIST NCls violates the octet nle:nitrogen n have no more than eight elec s (or four atoms)around it d orbitals in bonding,so PCls is a stable,isolable compound. 1-25 Your Lewis structures may look different from these.As long as the atoms are connected in the H HH H-N-N-H (b)H-N=N-H ()H-( ④I (a) Ha9 HH HHH 13 Copyright013 Pearson Education,Inc
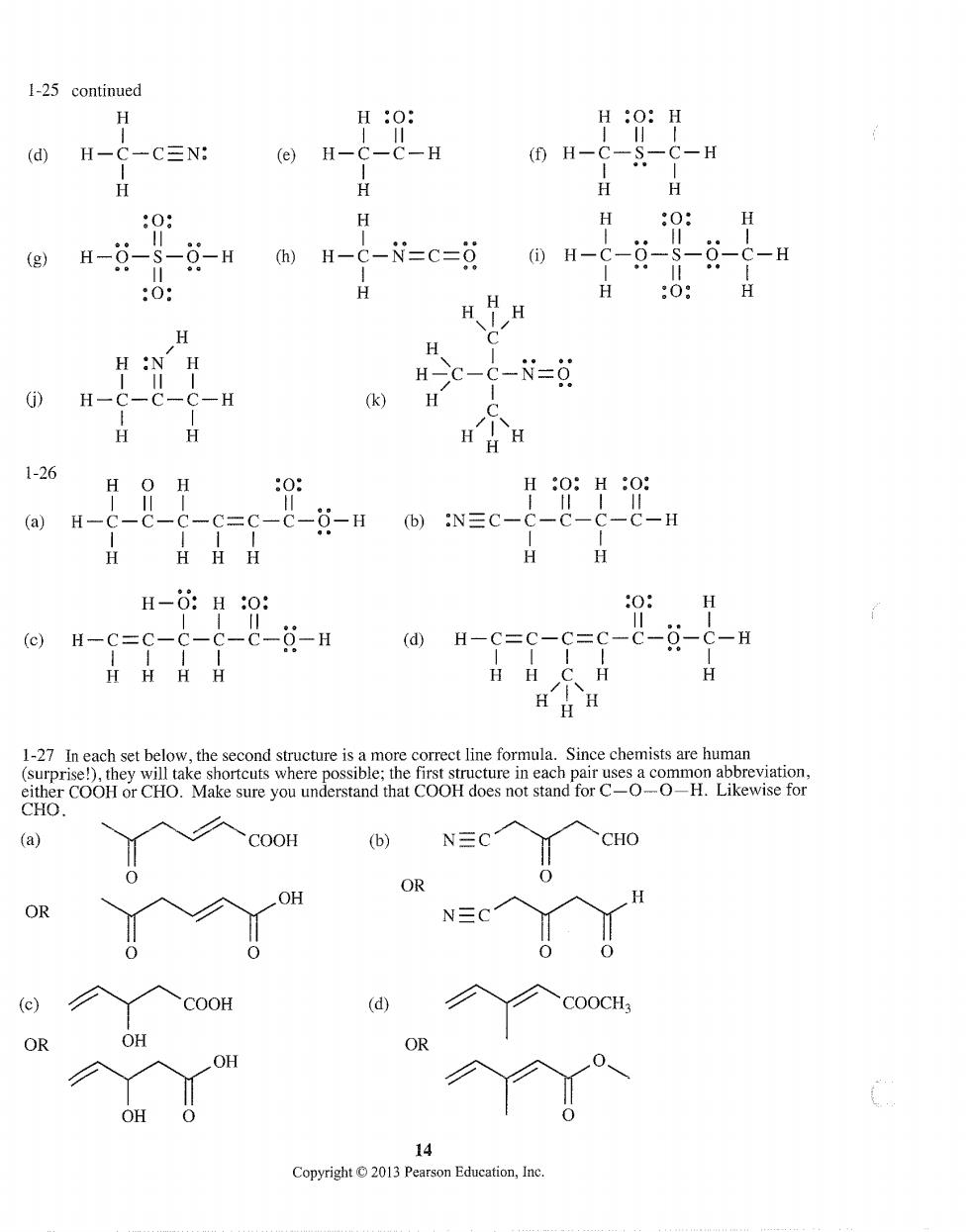
1-25 continued H H:O: C三N: H _C-H C-H H H H 0 0: (g H-- H-C-N=C=δ ①H-C-0- 0 -H HH H H :-N-0 H k H 126 H :O:H :O: (a) 9-H H-:H0: ()H-c=c-c-c-c-8-H (d)H-c=c-c=c-c-g-c-B HHH H 1-27 In each set below,the second structure is a more correct line formula.Since chemists are human COOH (b) CHO COOH (d) OR 14 Copyright2013 Pearson Education,Inc
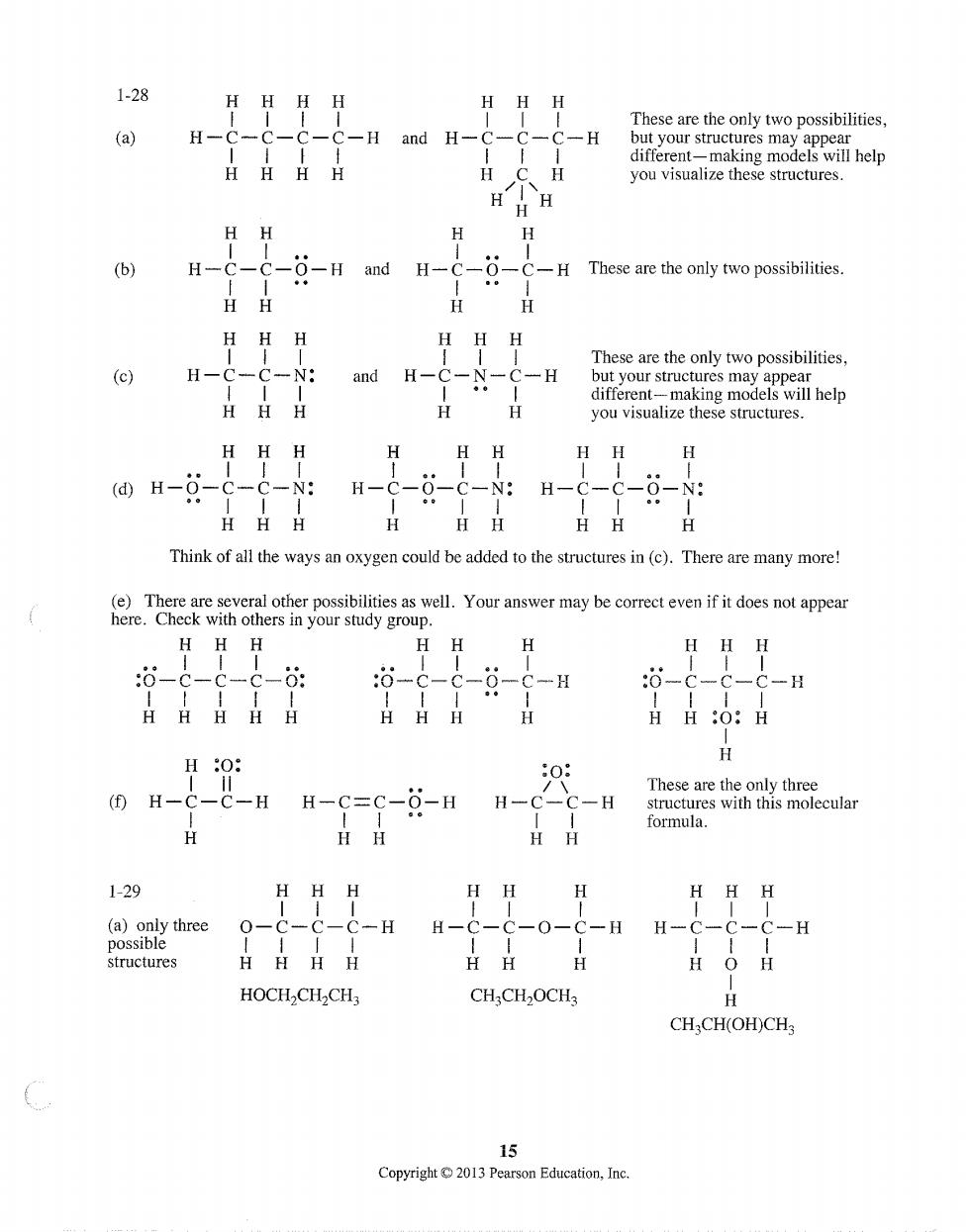
1-28 HHHH H HH (a) H- C-H and H-C- C-H t different-making models will help H H you visualize these structures. H (b) H-C -6-H and H-C-6- -C-H These are the only two possibilities. HHH HH H These are the only two possibilities. H-C-C-N:and H-C- -N -C-H but your structures ma appear HHH different- ing models will help HH H (d)H-6-c-C-N: H- c-0-c-N:H-c-C-0-N: HHH Think of all the eaded tothe in().Thereare many more HHH HH:O:H H-C=C-0-H H-c-c-H These are the only three structures with this molecular HH 1-29 HH H H一9 -0-9 H一 structures 。H HOCH2CH2CH3 CHCH2OCH CHCH(OH)CH 15 Copyright013 Pearson Education,Inc
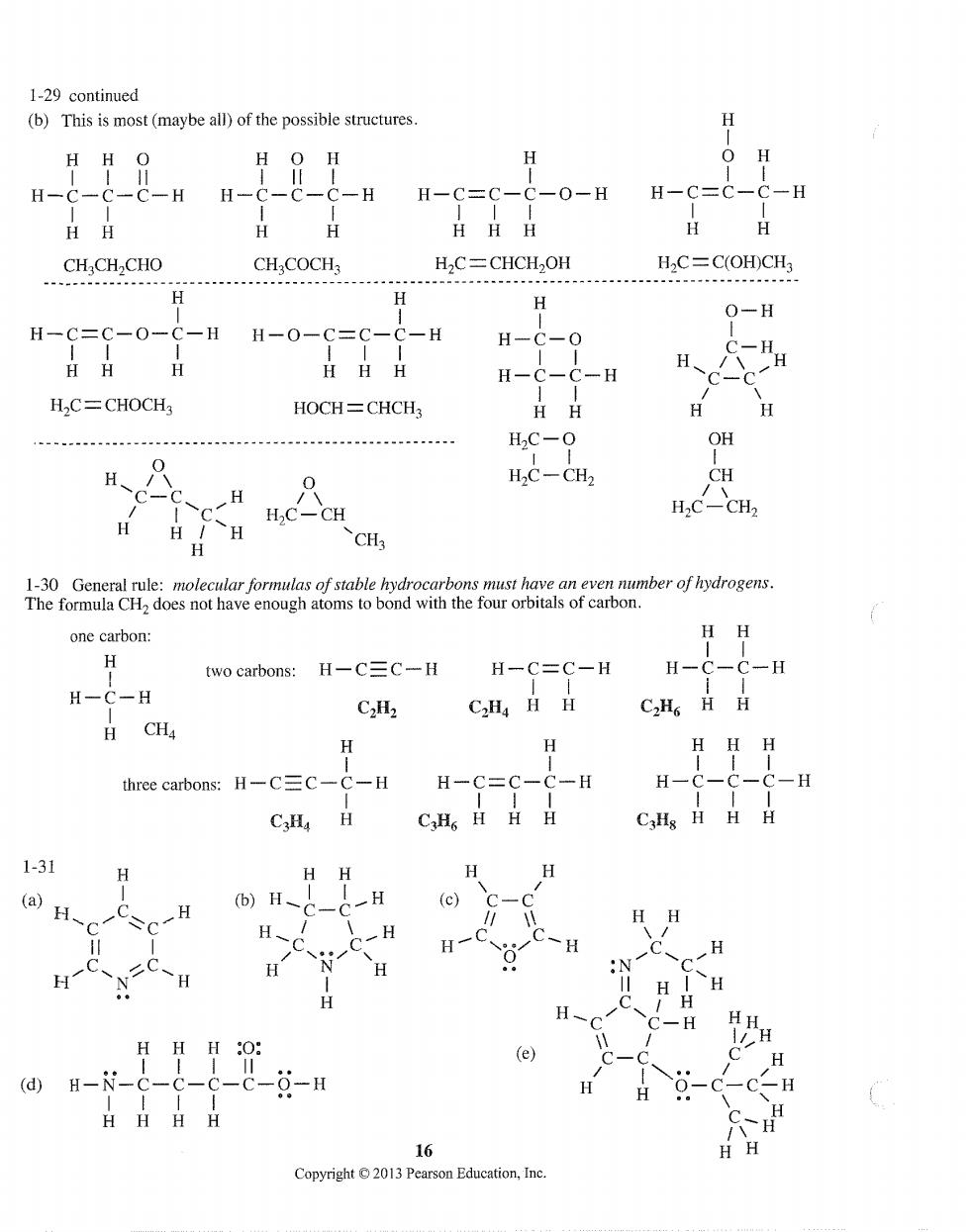
1-29 continued (b)This is most(maybe all)of the possible structures H H HO HO H H H-C--&-HH-C--C-H C-0-H H-C=C-C-E HH H CHCH CHO CH COCH H2C=CHCH2OH H2C=C(OH)CH H H 0-H H-C=C-0-C-H H-0-C=C-C-H H-C-0 H日H 11 H- HOCH=CHCH3 HH H H2C-0 OH H、 HC-CH CH H C H,C-CH H.C-CH H H CH one carbon: HH H two carbons:H-C=C-H H-C=C-H H-C-C-H -H C2H2 C.Ha HH C.HHH CH H HH H three carbons:H-C=C-C-H H-( -H H-C- C Hs HH H CaHs HH 1-31 (a)H- (b)H、 H、 H-C H H HHH :O: H (d)H--c HHHH 16 Copyright 2013 Pearson Education,Ine