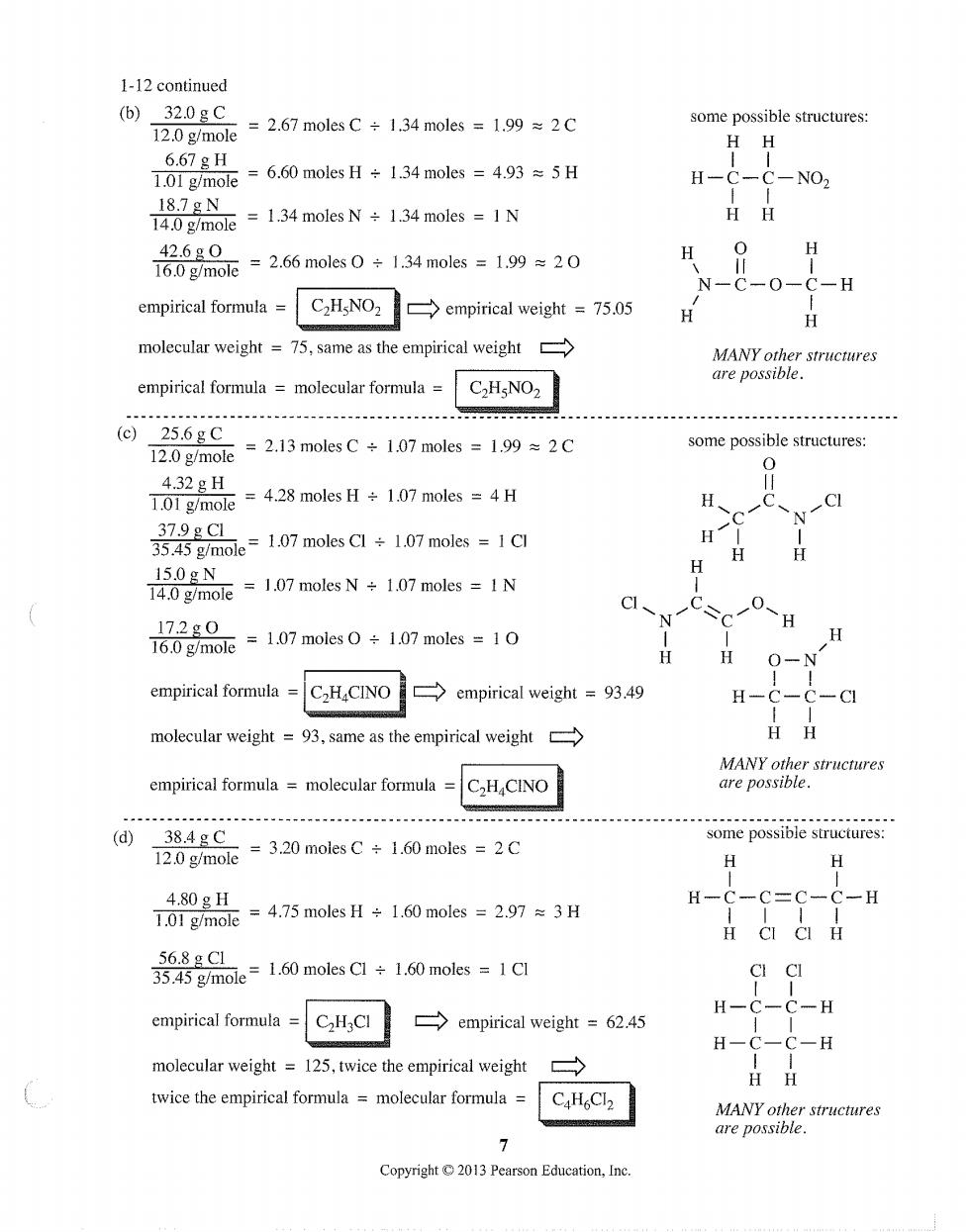
1-12 continued (b)32.0gC =2.67 moles C÷1.34 moles=1.99≈2C some possible structures: 12.0 g/mole H-C-C-NO2 m1moleN1 mlIN HH 760gm0=2.66 moles0÷134 moles=199=20 42.6g0 H N-C-0-C-H empirical formula C2HgNO2 empirical weight =75.05 H molecular weight=75,same as the empirical weight MANY other structures are possible. empirical formula molecular formula C.HsNO2 (c)25.6gC T2.0g/mole =2.13 moles C1.07 moles=1992C some possible structures: 4.32gH 101gm nole 4.28 moles H+1.07 moles =4 H CI 35.45 g/mole=1.07 moles Cl+1.07 moles =1Cl 37.9gC1 H H 140mole =1.07 moles N+1.07 moles =IN 15.0gN 16.0g/mole =1.07 moles1.07 moles =1 17.2g0 H H O-N empirical formula =C2HCINO empirical weight =93.49 H-C -CI molecular weight=93.same as the empirical weight HH MANY other structures empirical formula =molecular formula =CHCINO are possible. (d)38.4gC some possible structures: 12.0 g/mole =3.20 moles C 1.60 moles 2C H H 4.80gH 70gmoe=4.75 moles H÷1.60 moles=2.97≈3H 3g品e=160 moleC÷160nols=1d CI CI C-H empirical formula H C2H3CI empirical weight =62.45 molecular weight=125,twice the empirical weight twice the empirical formula molecular formula CaHCl2 MANY other structures are possible. 7 Copyright2013 Pearson Education,Ine

1-13 1 mole HBr (a)5.00g HBr x 80.9g HBr =0.0618 moles HBr 0.0618 moles HBr → 0.0618 moles HO+(100%dissociated) 0.0618 moles H,0+ 1000mL 0.618 moles H3O 100mL IL solution pH=-l0g1o[H30*]=-log1o(0.618)= 0209 (b)1.50g NaOH x 1 mole NaOH =0.0375 moles NaOH 40.0 g NaOH 0.0375 moles NaOH 0.0375 moles-OH (100%dissociated) 0.0375 moles-OH 1000mL 0.75 moles-OH =0.75M 50.mL. IL IL solution [H,0*1=Lx104 =1x10-14 【OH] =1.33x10-14 0.75 pH=-1og1o[H30*】=-log10(1.33x10-14)= 13.88 1.14 (a)By definition,an acid is any species that can donate a proton.Ammonia has a proton bonded to cid (although a very v par so ammonia is basic.In water,ammonia is too weak an acid to give up its proton:instead,it acts as a base and pulls a proton from water to a small extent. (b)water as an acid:H2O NH3OH NH4* water as a base:H2O HCI HO+Cl (c)Hydronium acting as an acid in water solution will have this chemical equation: H,0 +H0、三H,0+H0→K= [H,O][A】H3O*]H2O] =H20j HA A [HA] HO [H2O]= 1000 g H2O I mole H2O =55.55M=K3→pK2=-l0g(55.55)=-1.74 ILH O 18.0gH20 (d)methanol as an acid:CH2OH NH3 CH3O NH+ methanol as a base:CH3OH H2SO CH,OH2+HSO 8 Copyright2013 Pearson Education,Inc
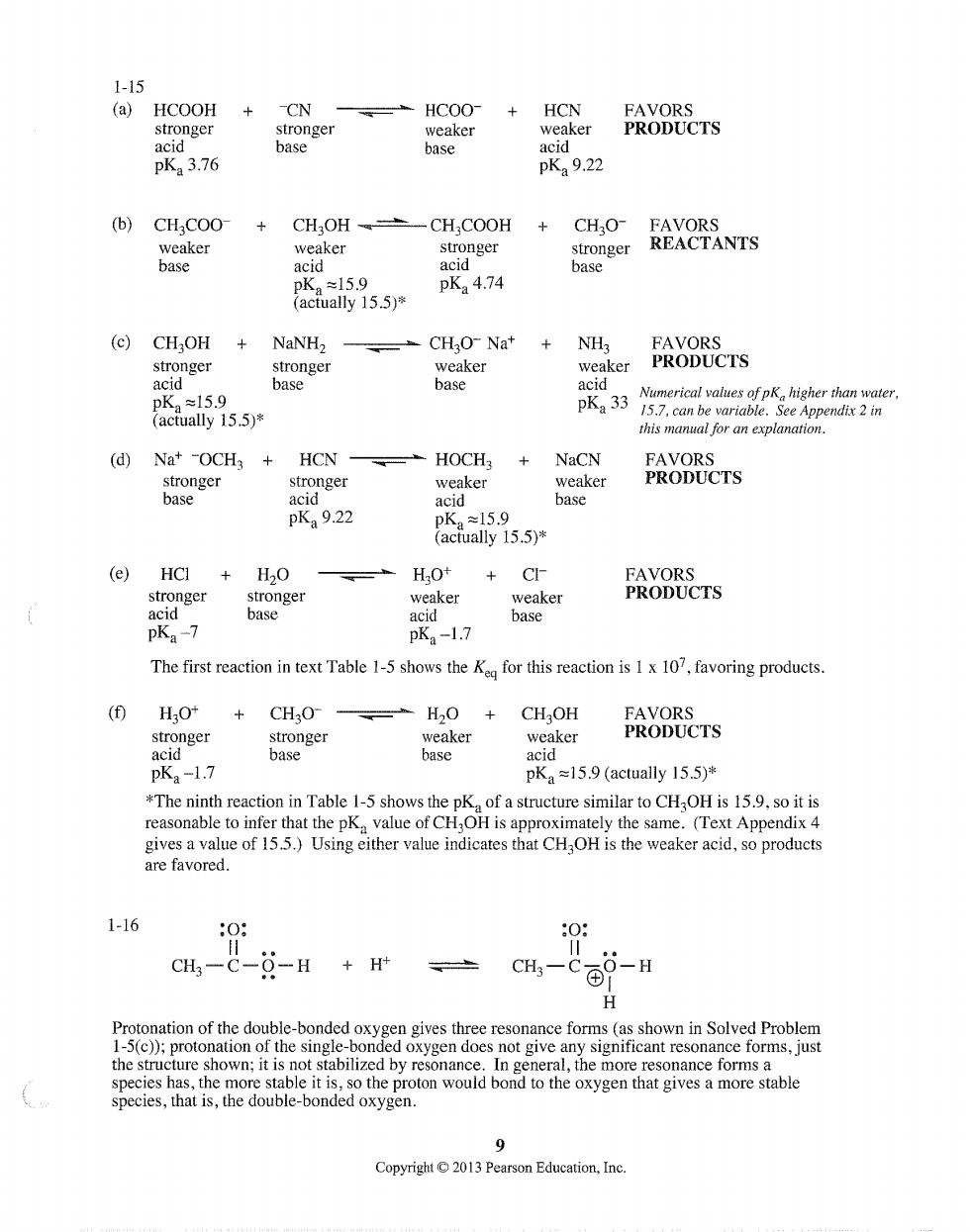
1-15 (a)HCOOH +CN =HC00 +HCN weaker PRODUCTS pK3.76 pKa 9.22 (b)CH;COO- -CH COOH CHO- stronger stronger REACTANTS ✉15.9 K4.74 (actually 15.5)* (c)CH3OH NaNHz CHO-Nat NH3 FAVORS stronger weaker weaker PRODUCTS base base 15.9 ctually 155)* this manal for an explanation. (d)Nat -OCH3 HCN --HOCH3 NaCN FAVORS stronger stronger weaker weaker PRODUCTS base acid acid base pKa9.22 (e)HCI+H2O CI- FAVORS stronger stronger weaker weaker PRODUCTS base acid base pKa-7 pKa-1.7 The first reaction in text Table 1-5 shows theK for this reaction is 1x 107,favoring products. (f)HO +CHO- =H20+ CHOH PRODUCTS base acid pKa-1.7 pKa =15.9 (actually 15.5)* *The ninth reaction in Table 1-5 shows the pKa of a structure similar to CHOH is 15.9.so it is reasonable to infer hat the pKa value of CH,OH is approximately the same.(Text Appendix 4 gives a value of 15.5.)Using either value indicates that CH OH is the weaker acid,so products are favored. 1-16 0 0 CH-C-0-H +H+ H ation of the double the structure shown:it ispot stabso the protoo would pond ne the monhesonance foomsable the oxygen that gives a more stable Copyright 9 2013 Pearson Education,Inc
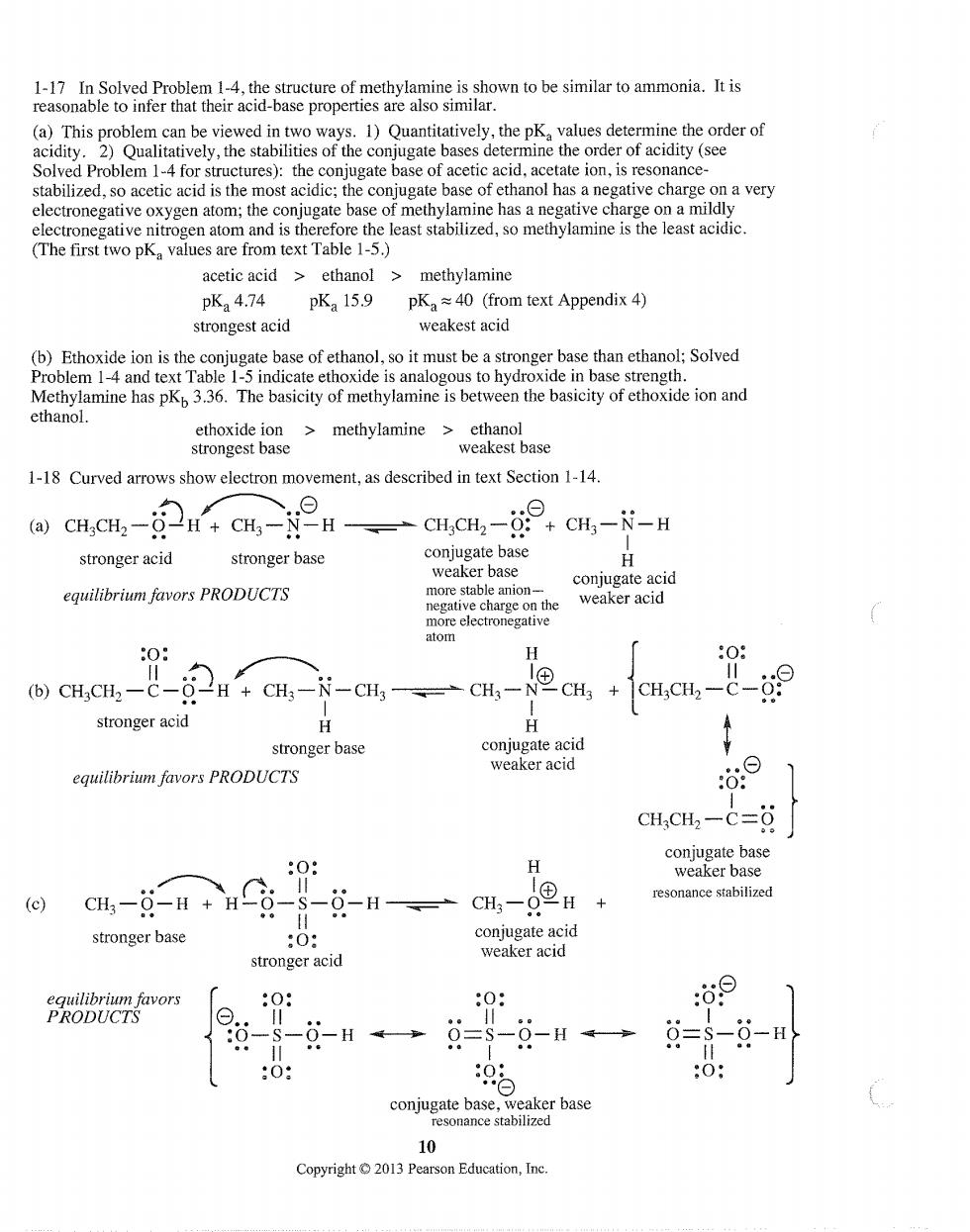
1-17 In Solved Problem 1-4,the structure of methylamine is shown to be similar to ammonia.It is reasonable to infer that their acid-base properties are also similar. rate bases determine the order of acidity (see Solved Problem 1-4 for structures):the conjugate base of acetic acid,acetate ion,is resonance- stabilized.so acetic acid is the most acidic:the conjugate base of ethanol has a negative charge on a very electronegative oxygen atom;the conjugate base of methylamine has a negative charge on a mildly electronegative nitrogen atom and is therefore the least stabilized,so methylamine is the least acidic. (The first two pK values are from text Table 1-5.) acetic acid>ethanol>methylamine pK24.74 pKa 15.9 pK40 (from text Appendix 4) strongest acid weakest acid (b)Ethoxide ion is the conjugate base of ethanol,so it must be a stronger base than ethanol;Solved Problem 1-4 and text Table 1-5 indicate ethoxide is analogous to hydroxide in base strength. Methylamine has pKp3.36.The basicity of methylamine is between the basicity of ethoxide ion and ethanol. ethoxide ion>methylamine cthanol strongest base weakest base 1-18 Curved arrows show electron movement,as described in text Section 1-14 CH.CH,CH-cH.cH CH-- stronger acid stronger base conjugat conjugate acid equilibrium favors PRODUCTS weaker acid 0: L (b)CHCH2一C-O CHs-N-CH- +cmcu,-- stronger acid H stronger base conjugate acid equilibrium favors PRODUCTS weaker acid CH.CH2-c=8 H resonance stabilized (c) CH,-g-H+H stronger base 0 conjugate acid stronger acid weaker acid 0 :0: 09 --H←→=-9-H→ g=-- 9.e :0: ) eaker base 10 Copyright01 Pearson Education,Ine
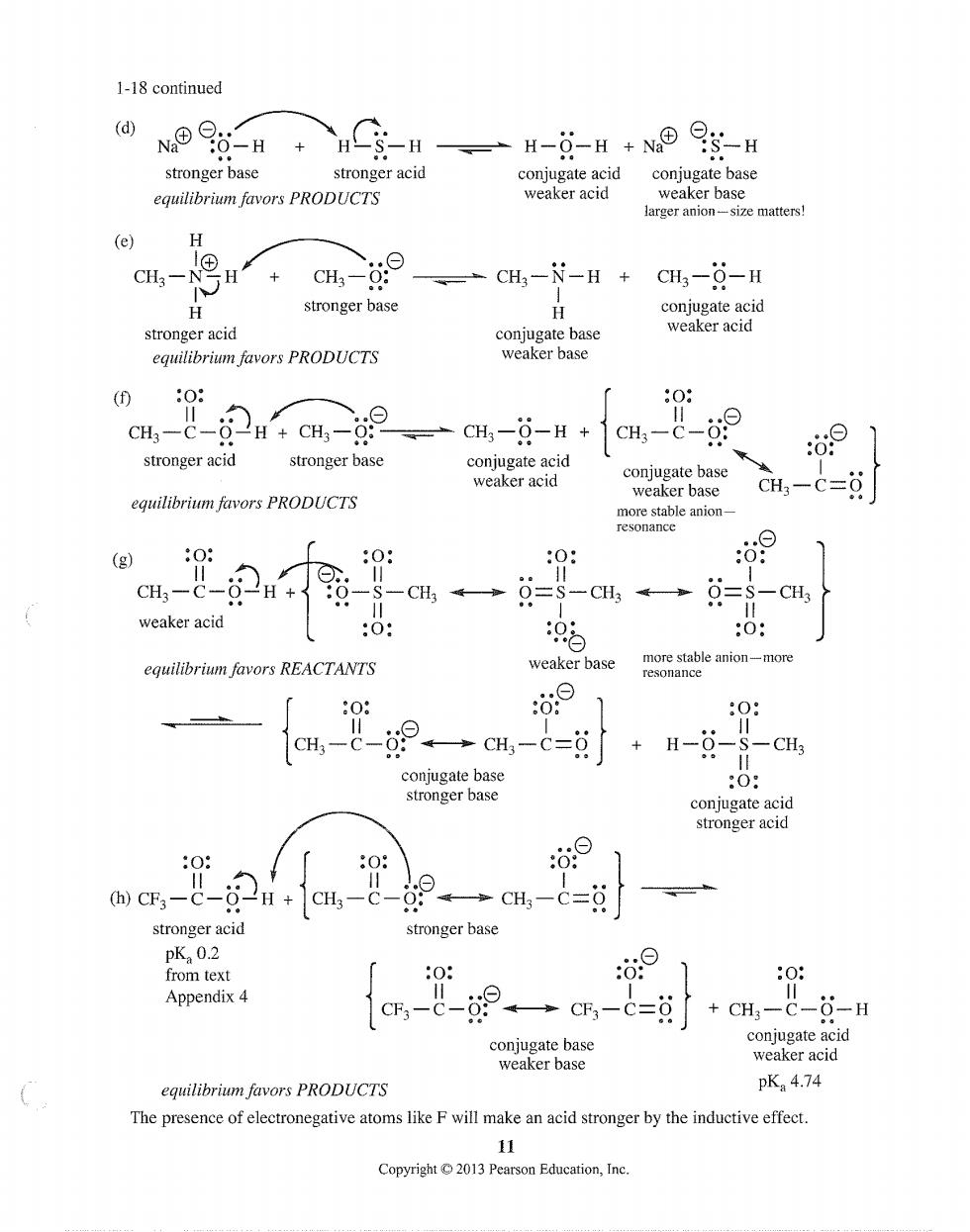
1-18 continued (d) N®a-H +C含-H一H-日-H+N0gH stronger base stronger acid conjugate acid conjugate base equilibrium favors PRODUCTS weaker acid ize matters H CH,- + CH3-0: 、=CH,-N-H+ CH3-6-H H stronger base H stronger acid conjugate base equilibrium favors PRODUCTS weaker base 08 0: H,-C-2HcHa9、一cH-g-H+ stronger acid conjugate acid -&-9 stronger base 0 weaker acid coniugate base equilibrium favors PRODUCTS weaker base CH-c=0 (g) :0: 0: :0: :d9 cH--246 -CH。→=s-CH→ =CH weaker acid :0: 05 :0: equilibrium favors REACTANTS weaker base le anion-more 0 00 1 : aH--89一cH-c=8+H-- -CH3 coniugate base stronger base :0: conjugate acid stronger acid 0 (h)CF-C- 2i+1em,- stronger acid stronger base pK0.2 from tex :0: ⊙ 0: Appendix 4 C,-C-→C,-C=g +CH-C-0-H conjugate base conjugate acid weaker base weaker acid equilibrium favors PRODUCTS pK:4.74 The presence of electronegative atoms like F will make an acid stronger by the inductive effect. Copyright C2013 Pearson Education,Inc