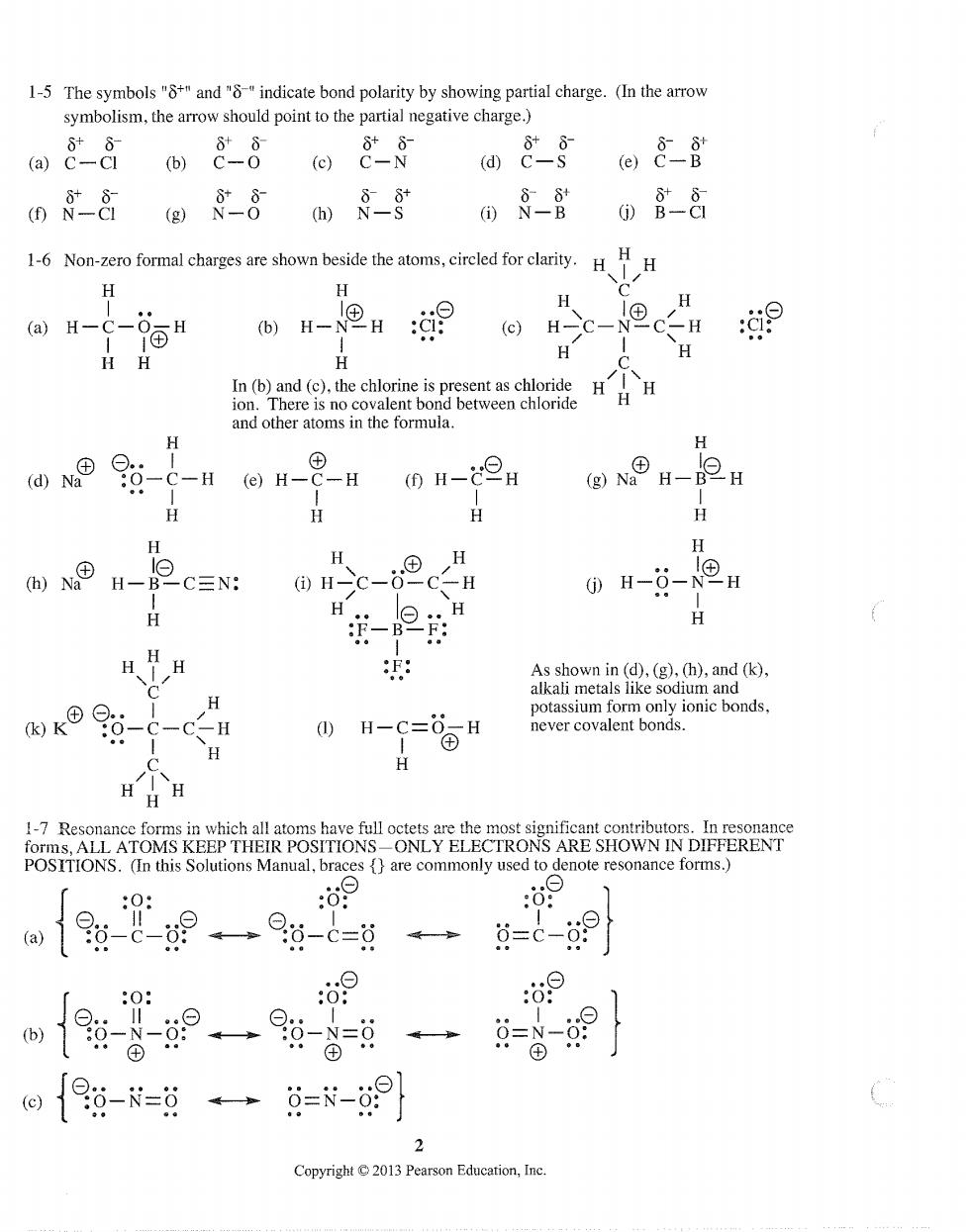
1-5 The symbols""and"indicate bond polarity by showing partial charge.(In the arrow symbolism,the arrow should point to the partial negative charge.) a8g e)8 m&@》8 08-80含8 16oinomleagsconhetceabns.cradortdbn.H其 H-H9 H (H-C-NCH 9 H 仙®--HH-9H (H- (GO NO H-H H H H H H H e H一B -C=N: H H--NH -2 H H H HH 测 As shown in (d),(g).(h),and (k). H alkali meta H H enote resonance forms. 0 09 a19-8-9一9-c=过 4-=c-9 :0: 6 e{6-=日日=-] Copyright2013 Pearson Education,Inc
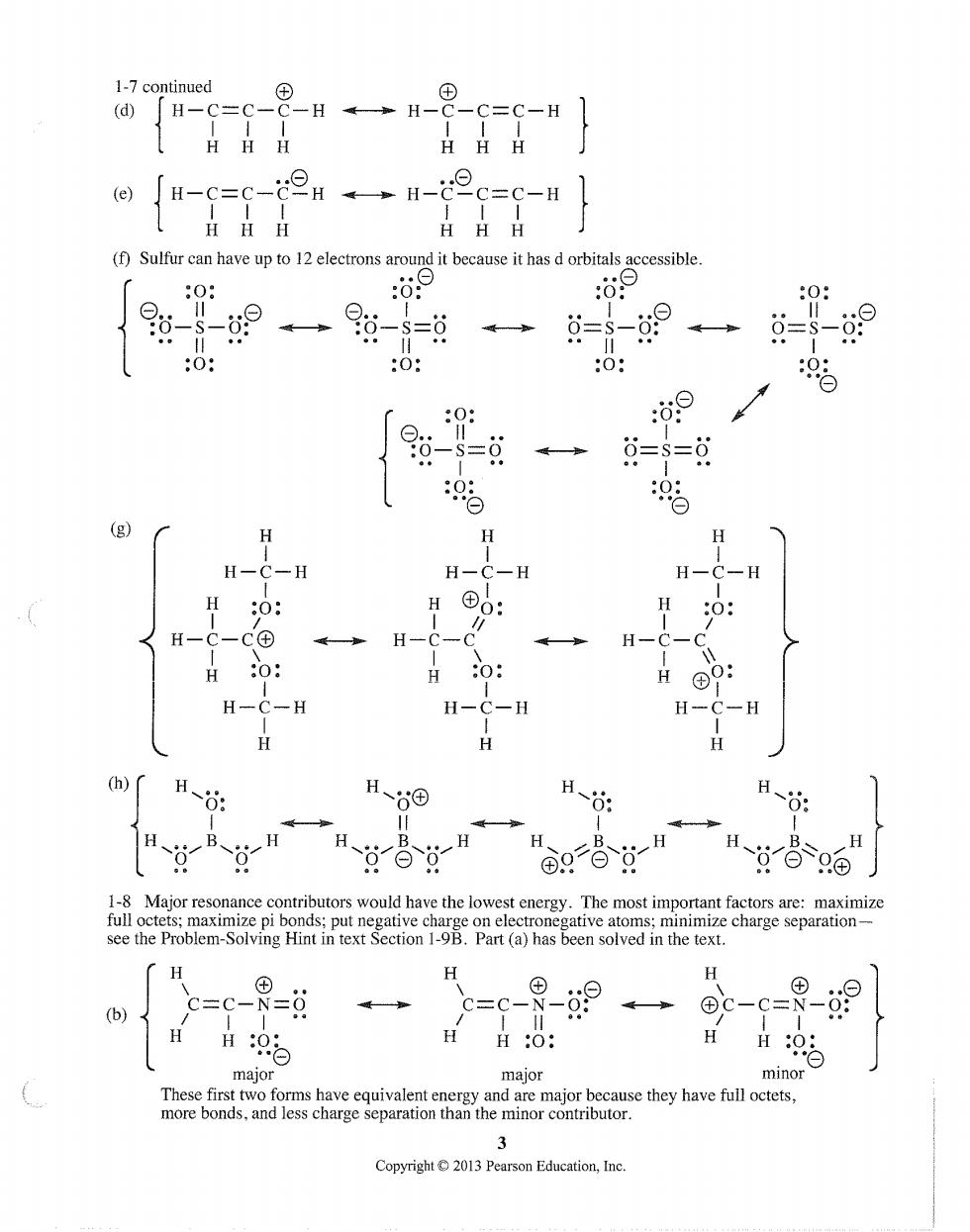
1-7 ontinued (d) HHH (f)Sulfur can have up to 12 electrons around it because it has d orbitals accessible 0: :o9 0: = 0: 0: 6=S=6 H-C-H H-O 0: (h) 1-8 Maior reson ce contributors would have the lowest The most in e te mhote ome H c=c-9= H H H98 major major minor asa9getchoyaeocaea than the minor contributor Copyright013 Pearson Education,Ine
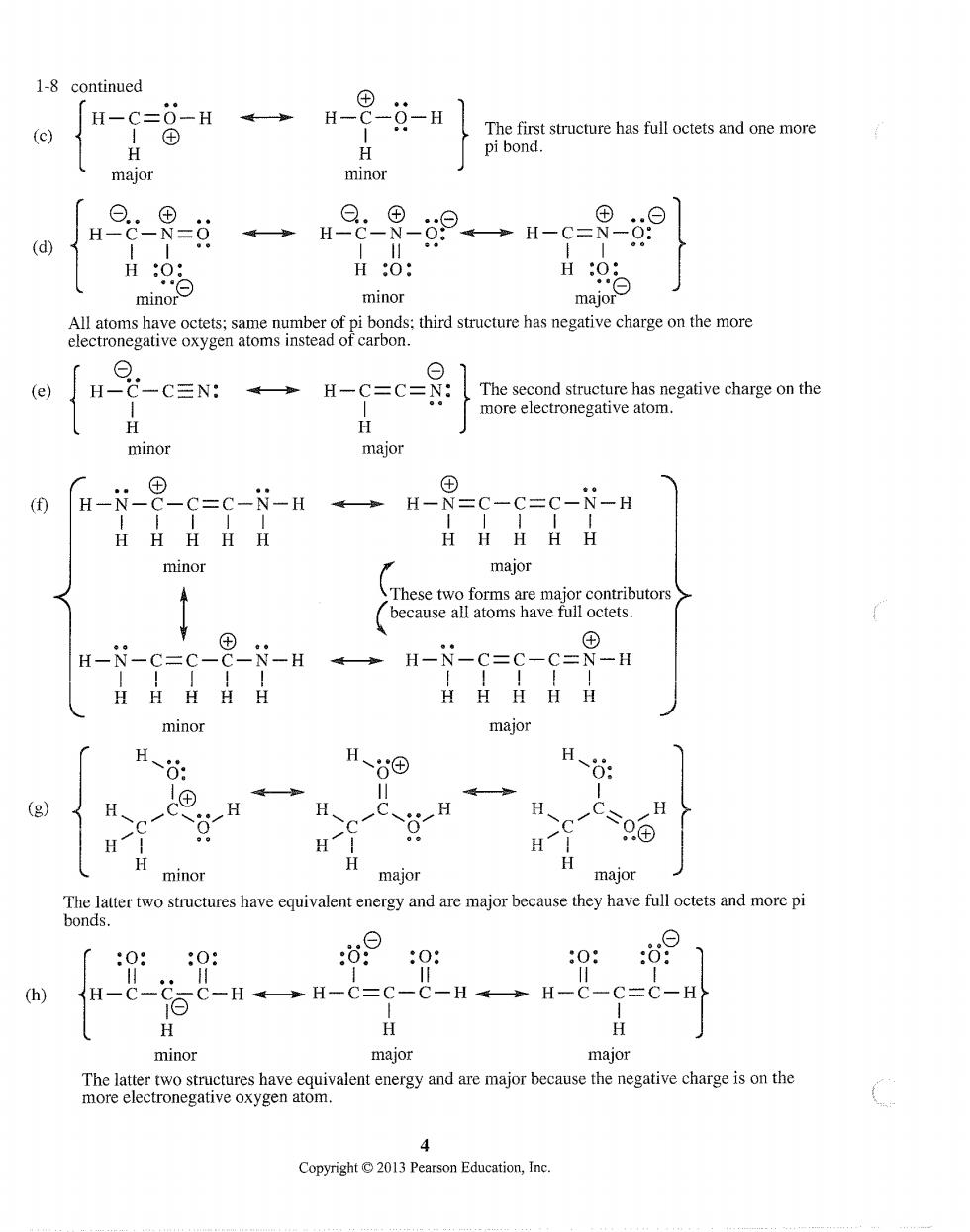
1-8 continued 「H-c=-H→H-8-g-H (c) The first structure has full octets and one more pi bond. minor 9→H-c=9- (d) minor major havecmbonds:thsr has negative charge electronegative oxygen atoms instea H-C-CEN: H-C=C=N: The second structure has negative charge on the more electronegative atom. minor major H-N_ -c=C-N-H→ H HHHHH minor major H-N-C=c- -H4→ HHHHH minor major H、 H、⊙ 日-6 H H H、 H H H H minor major major The latter two structures have equivalent energy and are major because they have full octets and more pi bonds. 0 99 (h) H-C-C_ -H→H-C=c-C-H→H-C-C=C-H H H H minor major major The latter two structures have equivalent energy and are major because the negative charge is on the more electronegative oxygen atom. Copyright013 Pearson Education,Ine
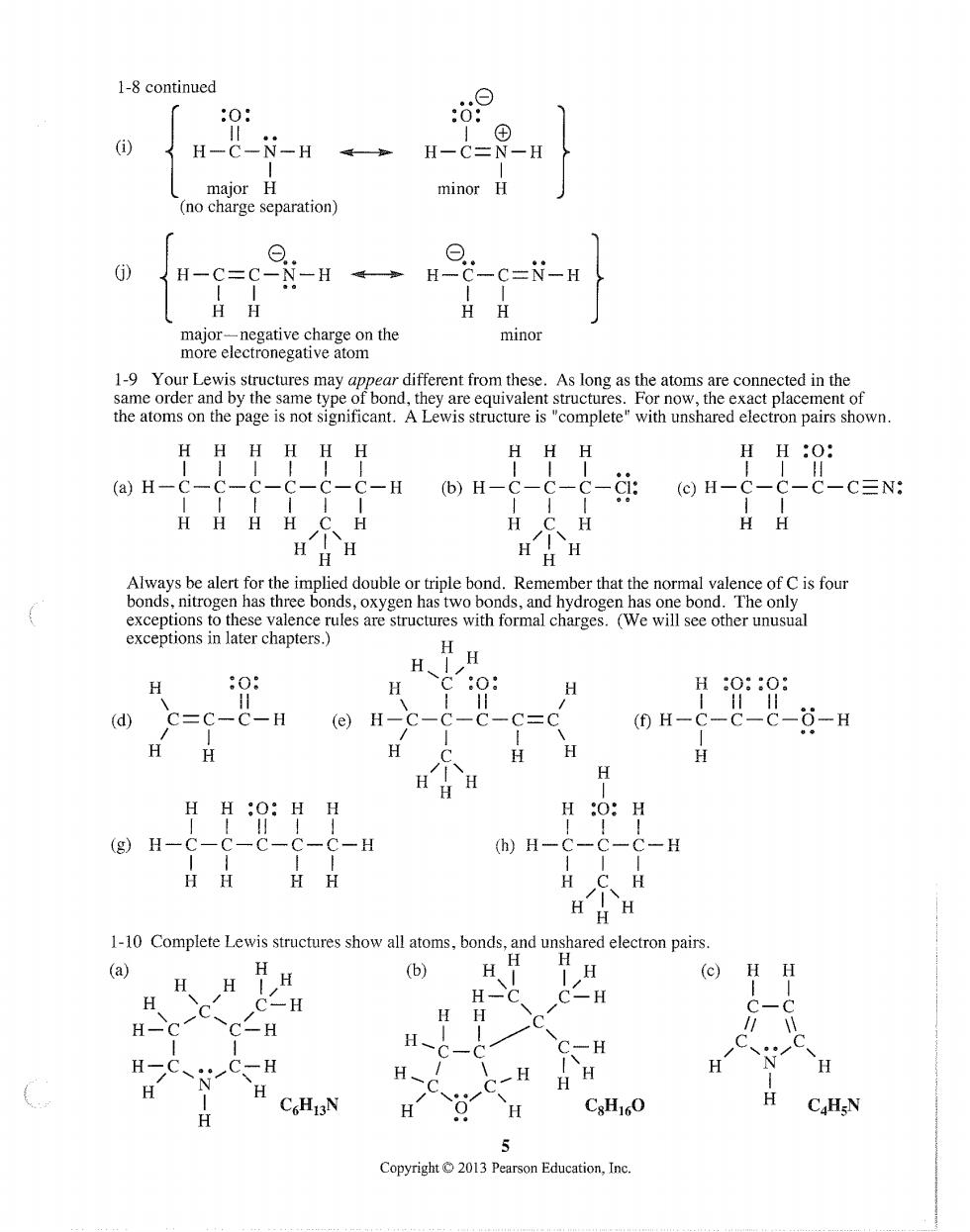
1-8 continued :0: 0⊙ H-C-N-H H-C=N-H major H minor H (no charge separation) 0 H-C=C--H H -C-N-H HH major-negative charge on the minor more electronegative atom different from thes As lor the atoms on the page is not significant.A Lewis structure is"complete"with unshared electron pairs shown HH HH HH HH·O (a)H-C- C-C-C-C-C-H H- ⊙H- -C-CN: HHHH C H H HH H H H 6S6 ydrogen ws one bond The charges h er unusu HH H、 (e)H-c-c-c-c=c (0H-c-c-c-0-H H H H:0:H (h)H-C-C-C-H H C H H H H 1-10 Complete Lewis structures show all atoms,bonds,and unshared electron pairs (a) (b) H (c)HH H C-H 一H C-H H- C-H N ,一H HH H H H CHI3N H CsH16O 1 CHN 5 Copyright 2013 Pearson Education.Inc
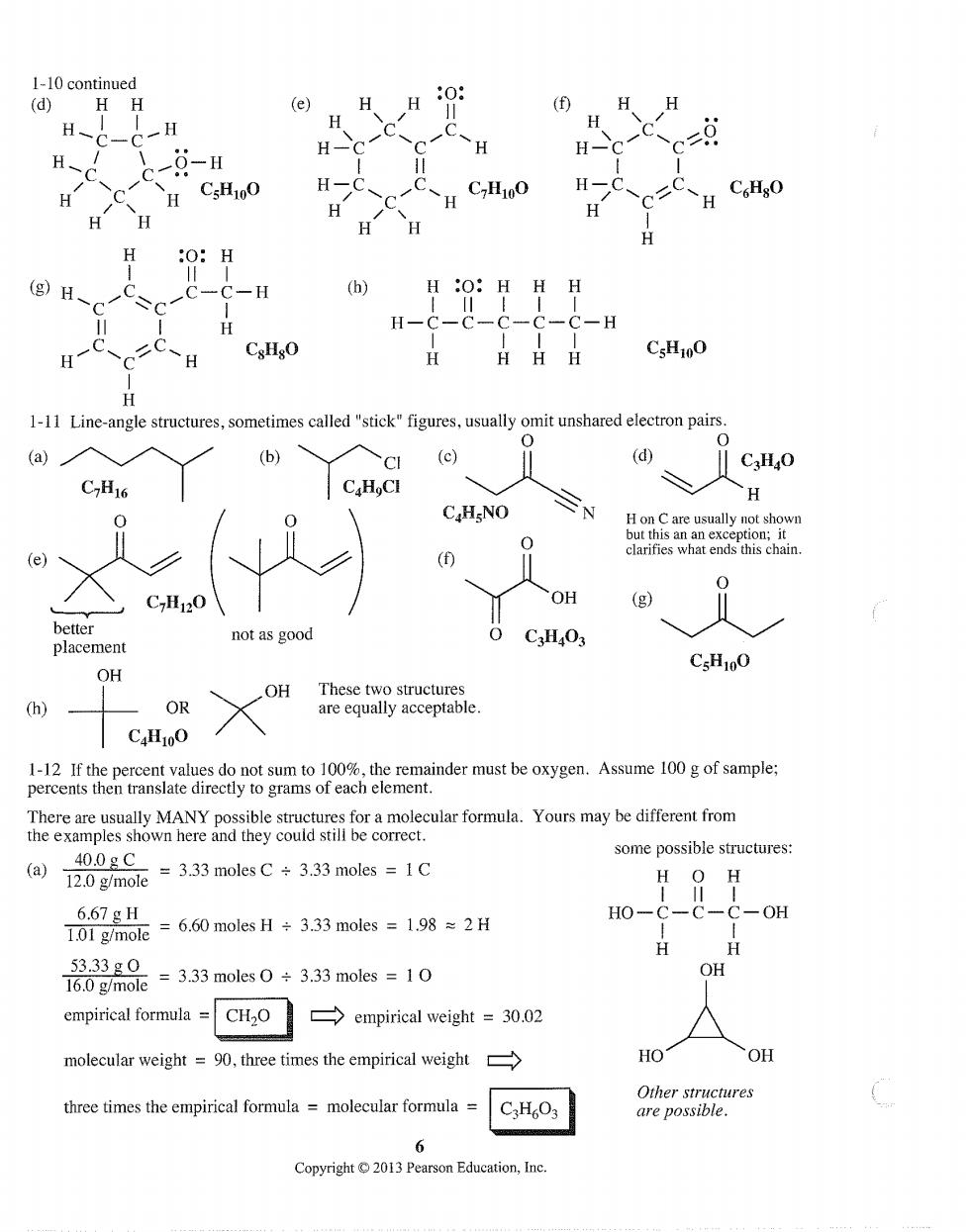
1-10 continued (d) HH (e) H H H- 0-H g)H. C.HO H日H CsH1oO 1-11 Line-angle structures,sometimes called"stick"figures,usually omit unshared electron pairs. (b) cI (c) ‖CgHO C7H16 H H on C are ends this chair C7H120 OH (g) tter not as good C3HO3 placement OH CsH100 OR CaH10O remainder must be oxygen.Assume 100g of sample; ams of each element. There are usually MANY possible structures for a molecular formula.Yours may be different from the examples shown here and they could still be correct. 40.0gC some possible structures: (a)2.0 g/mole 3.33 moles C 3.33 moles 1C H 6.67gH 101gym0c=6.60 molesH÷3.33 moles=1.98≈2H HO-C-C-C-OH 16.0 g/mole =3.33 molesO+3.3 moles =10 53.33g0 OH empirical formula=CHO empirical weight 30.02 molecular weight =9,three times the empirical weight HO OH three times the empirical formula=molecular formula Other structures C3H6O3 are possible. Pearson Education,Ine