
SYMBOLS AND ABBREVIATIONS text.Do not expect all of these to make sense to the textbook by W you now.I your study of organic chemistry) BONDS a single bond a double bond a triple bond a bond in three dimensions.coming out of the paper toward the reader a bond in three dimensions.going behind the paper away from the reader a stretched bond,in the process of forming or breaking ARROWS in a reaction,shows direction from reactants to products signifies equilibrium(not to be confused with resonance) signifies resonance(not to be confused with equilibrium) shows direction of electron movement: the arrowhe with one the arrowhead with two barb 十 shows polarity of a bond or molecule,the arrowhead signifying the more negative end of the dipole SUBSTITUENT GROUPS Me a methyl group,CH 号 an ethyl group,CH2CH3 Pr a propyl group,a three-carbon group (two possible arrangements) a butyl group,a four-carbon group(four possible arrangements) R the general abbreviation for an alkyl group(or any substituent group bonded at carbon) Ph a phenyl group,the name of a benzene ring as a substituent.represented Ar the general abbreviation for an aromatic group continued on next page vii Copyright013 Pearson Education,Ine
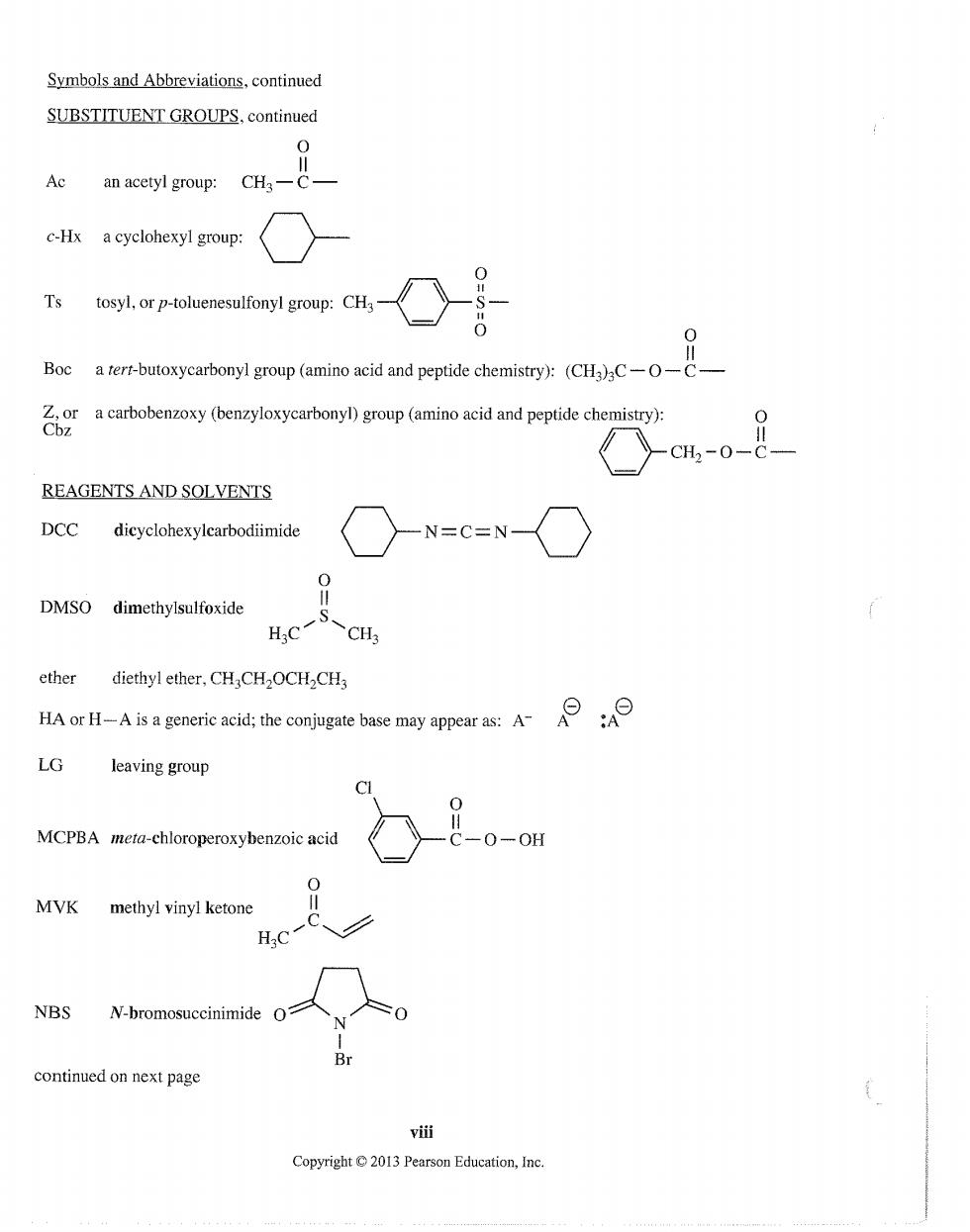
Symbols and Abbreviations,continued SUBSTITUENT GROUPS.continued an acetyl group: CHa-C :-Hx a cyclohexyl group: Ts Boc a rert-butoxycarbonyl group (amino acid and peptide chemistry):(CHa)C-- a carbobenzoxy(benzyloxycarbonyl)group(amino acid and peptide chemistry) -cH2-0-C REAGENTS AND SOLVENTS DCC -N=C=N- DMSO dimethylsulfoxide ether diethyl ether,CHCH2OCHCH generic acid:the conjugate base may appear as:A LG leaving group MCPBA meta-chloroperoxybenzoic acid -0-0 MVK methyl vinyl ketone H:C NBS W-bromosuccinimide continued on next page vi进 Copyright 2013 Pearson Education,Inc
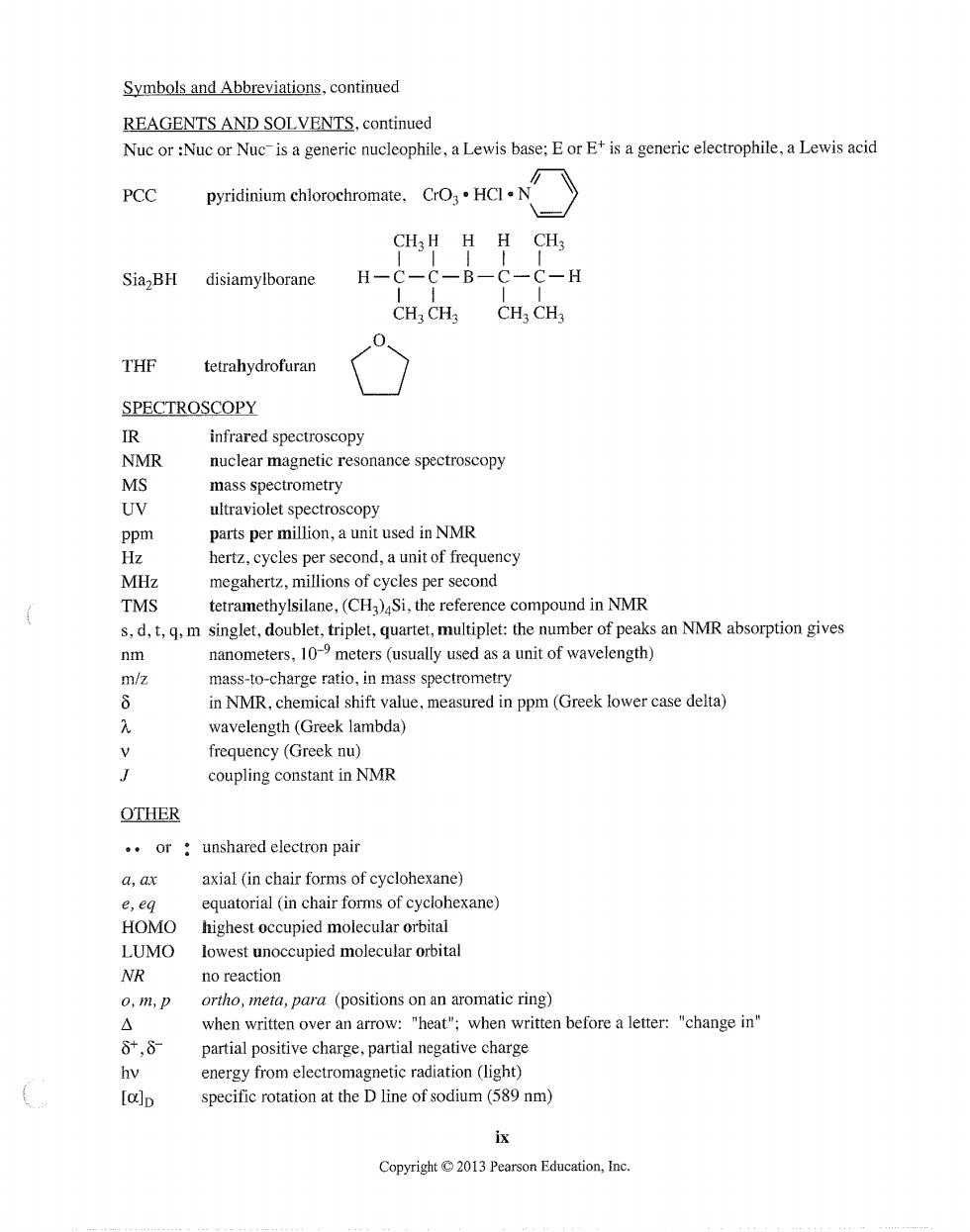
Symbols and Abbreviations,continued REAGENTS AND SOLVENTS.continued Nuc or:Nuc or Nuc-is a generic nucleophile,a Lewis base:Eor E+is a generic electrophile,a Lewis acid PCC pyridinium ehlorochromate.CrOHCIN CH3H HH CH3 SiazBH disiamylborane H -H CH:CH CH CH3 0、 THF tetrahydrofuran SPECTROSCOPY IR infrared spectroscopy NMR nuclear magnetic resonance spectroscopy MS mass spectrometry ultraviolet spectroscopy parts per million,a unit used in NMR MHz megahertz,millions of cycles per second TMS tetramethylsilane,(CH3)Si,the reference compound in NMR s.d.tm singlet,doublet,triplet,quartet,multiplet:the number of peaks an NMR absorption gives nm nanometers,10meters(usually used as a unit of wavelength) m/z mass-to-charge ratio,in mass spectrometry in NMR,chemical shift value,measured in ppm(Greek lower case delta) wavelength(Greek lambda) v frequency(Greek nu) coupling constant in NMR OTHER .or unshared electron pair a.ax axial (in chair forms of cyclohexane) e,eq equatorial (in chair forms of cyclohexane) HOMO highest occupied molecular orbital LUMO lowest unoccupied molecular orbital NR no reaction 0,m,p ortho,meta,para (positions on an aromatic ring) △ when written over an arrow:"heat";when written before a letter:"change in" 8,8 partial positive charge,partial negative charge energy from electromagnetic radiation (light) [a]p specific rotation at the D line of sodium(589 nm) ix Copyright2013 Pearson Education,I

Students:Add your own notes on symbols and abbreviations. Copyright013 Pearson Education.Inc
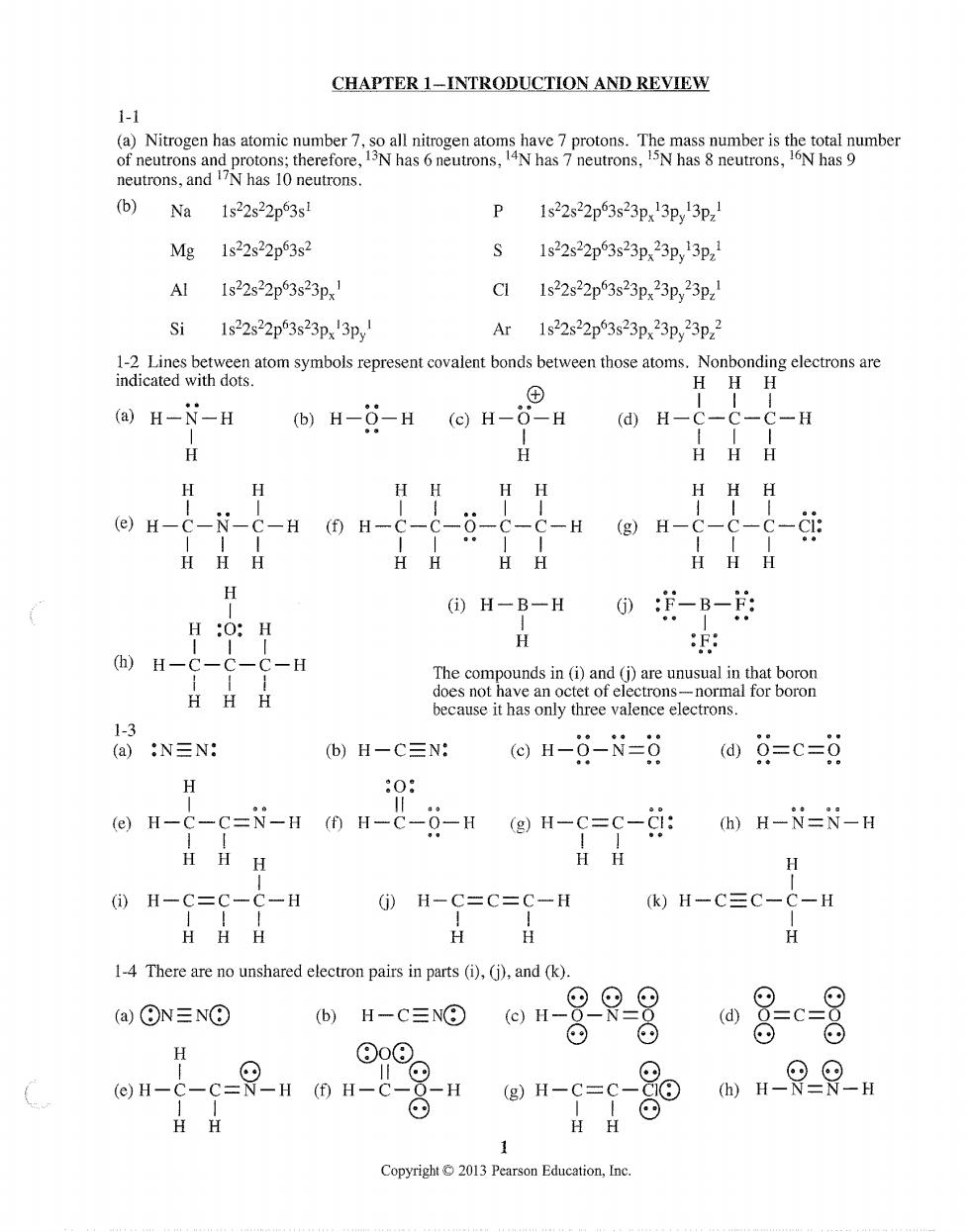
CHAPTER 1-INTRODUCTION AND REVIEW 1-1 (b)Na 1s22s22p3s! P1s22s22p3s23p,13p,13p, Mg 1s22s22p63s2 S1s22s22p63s23p,23p,13p, Al 1s22s22p3s23p C11s22s22p63s23p,23p,23pz Ar 1s2s2p3s23p,23p,23p. 品ahnmyamobprocmcowaaboahetwoatocabasnrme6kros6r HHH (a)H-N-H (b)H-0-H (e)H-6-H (d)H-C-C-C-H HHH H HH HH HHH @H-C---H0H-C-----H®H -c-ci: HHH HHHH HHH H 0H-B-H0:-B-的 H:O:H 四H----H because it has only three valence electrons. 1-3 (a):N≡N: b)H-C三N: ⊙H-9-N=日@Q=c=g H (H-c-C=-H (0 H-c-g-H (g)H-c=c-Ci:(H-N=N-H HHH HH (i)H-C=C-C-H H-C=C=C-H k)H-C三C-C-H H 1-4 There are no unshared electron pairs in parts (i),(j),and (k). (a)⊙N=NO 四-C三O因H-是只8 g (e)H- H(H-C H 11 n-6=6-80-9 HH Copyright2013 Pearson Education,Ine