
Unique property of carbon ---Bond to one another in a virtually limitless number of arrangements Carbon Atomic Weight 12.011 Melting Point 3823.2K Boiling Point 1st lonization Energy 1086.4 kJ/mol Density 2.266gcm3 Electron Configuration [He]2s22p2 Atomic Radius 77.2
Unique property of carbon ---Bond to one another in a virtually limitless number of arrangements
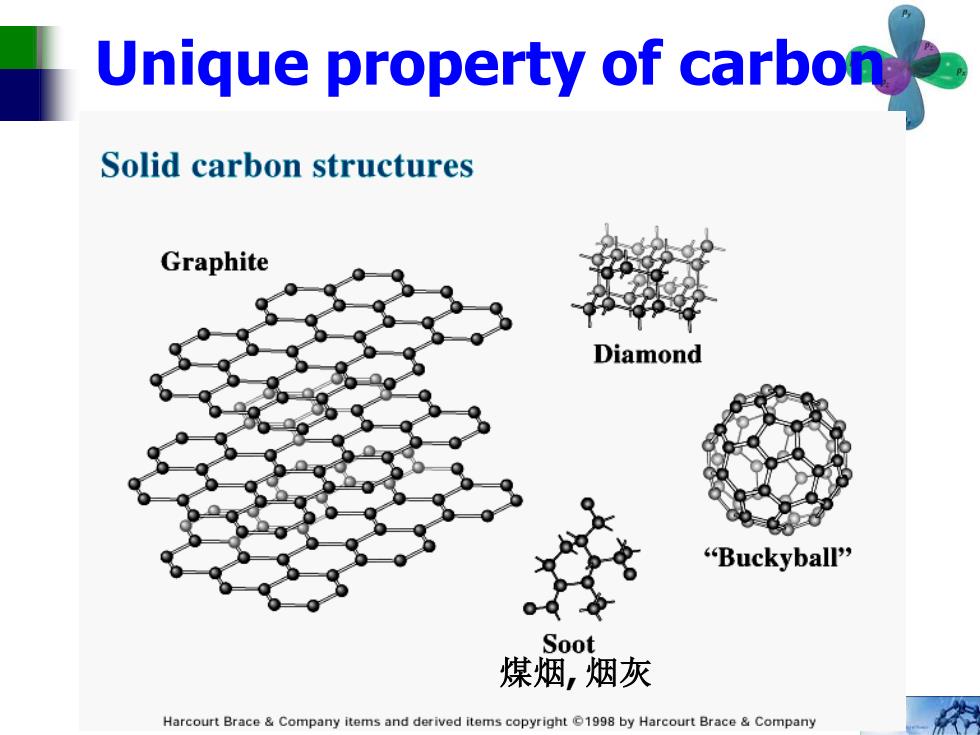
Unique property of carbon Solid carbon structures Graphite Diamond "Buckyball" Soot 煤烟,烟灰 Harcourt Brace Company items and derived items copyright 1998 by Harcourt Brace Company
Unique property of carbon 煤烟, 烟灰

Chemical Fullerene-A New Form of Carbon Connections A favorite chemistry examination question is:What are ists refer to it as Cea or fullerene:some call it "bucky- the elemental forms of carbon?For a long time,the an- ball"Kroto,Smalley,and Robert F.Curl were awarded swer was that pure carbon is found in two forms:graph- the 1996 Nobel prize in chemistry for this work.Some ite and diamond.These forms have been known for other fullerenes,such as C and Cst have now been iso centries,and it was generally believed that they are the lated and studied as well. only forms of carbon having extended nenworks of C at- Fullerenes have a rich chemistry.They behave as if oms in well-defined structures.But that is not so!The they have electron-deficient double bonds.Many differ scientific world was startled in 1985 when Richard Smal- ent fullerene adducts with a variery of structures have ley of Rice University and Harry W.Krot of the Univer- been prepared.Cationic derivatives of these adducts, sity of Sussex,UK.and their coworkers announced thar for example,bind tightly to DNA and can be used to they had detected a new form of carbon with the mo visualize it by clectron microscopy. lecular formula Cse They suggested that the molecule An important development in this field has been the has a structure that resembles a soccer ball;it has 1?five preparation of single-wall nanotubes,which are based on memnbered rings and 20 six-membered rings arranged Co or higher fullerenes and are extended for a very long such that each five-membered ring is surrounded by distance to make long molecules that are hundreds of six-membered rings.This structure reminded its dis times stronger than steel and can act as molecular wires. coverers of a geodesic dome.a sticture invented by Nanotubes can be used as the probe (a very sharp tip) the innovative American engineer and philosopher R in atomic force microscopes,which can be used to im Buckminster Fuller.Therefore,the official name of this age single molecules.The nanotubes make the sharpest allotrope of carbon has become fullerene.Most chem- possible tips because they are of molecular dimensions. Nanotubes are also playing an increasingly important role in the field of nanotechnology,indluding applica- tions in the area of molecular electronics.Nanotubes can be superconducting or semiconducting.depending on the exact structure of the nanotube
ORGANIC CHEMISTRY (PRIORTO1828) ORIGINALLY CHEMISTRY OF COMPOUNDS DERIVED FROM PLANT AND ANIMAL (ORGANIC)SOURCES 'CHEMISTRY OF LIFE
ORGANIC CHEMISTRY ORIGINALLY : CHEMISTRY OF COMPOUNDS DERIVED FROM PLANT AND ANIMAL (“ORGANIC”) SOURCES “CHEMISTRY OF LIFE” ( PRIOR TO 1828 )

History... IIn 1770,Swedish chemist Torberm Bergman was the first to express the difference between "organic"and "inorganic"substances Organic---- derived from living organism Originally,Study of compounds extracted from living organisms and their natural products INORGANIC CHEMICALS were found primarily in the earth as mineral deposits,but could also be prepared by man
History… ◼In 1770, Swedish chemist Torberm Bergman was the first to express the difference between “organic” and “inorganic”substances Organic ---- ◼ derived from living organism Originally, Study of compounds extracted from living organisms and their natural products INORGANIC CHEMICALS were found primarily in the earth as mineral deposits, but could also be prepared by man