
Bimolecular Reactions Example:CI+H2->HCI H Suppose we detect HCl in CI/H2 beam experiment: -if Cc in beam is doubled,then we find the no.of HCI molecules seen per second is also doubled. -similarly,doubling C2 doubles no.of HCI molecules Hence dCHcldt=kCclCH All bimolecular reactions are usually 2nd-order PDF文件使用"pdfFactory Pro”试用版本创建断fineprint.com,c里
Example: Cl + H2 ® HCl + H Suppose we detect HCl in Cl/H2 beam experiment: - if CCl in beam is doubled, then we find the no. of HCl molecules seen per second is also doubled. - similarly, doubling CH2 doubles no. of HCl molecules • All bimolecular reactions are usually 2nd-order Bimolecular Reactions Hence dCHCl/dt = kCClCH2 PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn 饦ÿ

For Elementary Reactions: aA+bB=IL+mM -dCldt=kC aCgb The molecularity is consistent with stoichiometric coefficient .The molecularity usually given by the order of the elementary reaction The order can only be 1,2,and 3. PDF文件使用"pdfFactory Pro”试用版本创建fm,fineprint.com,cn
aA+bB=lL+mM -dCA /dt=kCA aCB b · The molecularity is consistent with stoichiometric coefficient ·The molecularity usually given by the order of the elementary reaction · The order can only be 1,2,and 3. For Elementary Reactions: PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn _
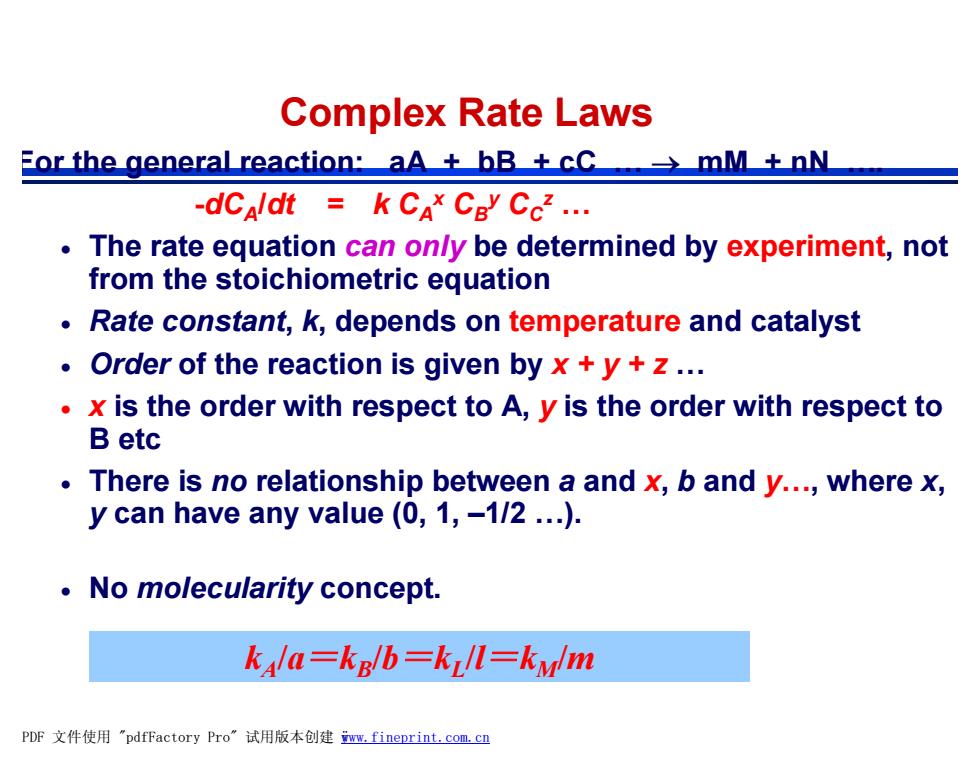
Complex Rate Laws For the general reaction:aA±bB土cCmM土nW -dCaldt k CAx CBy Ccz... The rate equation can on/y be determined by experiment,not from the stoichiometric equation Rate constant,k,depends on temperature and catalyst Order of the reaction is given by x+y+z... x is the order with respect to A,y is the order with respect to Betc There is no relationship between a and x,b and y...,where x, y can have any value (0,1,-1/2...). No molecularity concept. kala=kg/b=kll=kylm PDF文件使用"pdfFactory Pro”试用版本创建wm,fineprint,com,cn
For the general reaction: aA + bB + cC … ® mM + nN …. -dCA /dt = k CA x CB y CC z … · The rate equation can only be determined by experiment, not from the stoichiometric equation · Rate constant, k, depends on temperature and catalyst · Order of the reaction is given by x + y + z … · x is the order with respect to A, y is the order with respect to B etc · There is no relationship between a and x, b and y…, where x, y can have any value (0, 1, –1/2 …). · No molecularity concept. Complex Rate Laws kA /a=kB /b=kL /l=kM/m PDF 文件使用 "pdfFactory Pro" 试用版本创建 ÿwww.fineprint.com.cn

H2+Br2=2HBr -dCH2/dt=kCH2CBr211+KCHBr/CBr2 This rate equation has no order of the reaction. The rate law in terms of partial pressures dPA=kpPA dt dt kp=kc(RT)1-n or kc=kp(RT)n-1 PDF文件使用"pdfFactory Pro”试用版本创建f.fineprint.com,c迎
H2+Br2=2HBr -dCH2 /dt=kCH2CBr2 1/2/1+k’CHBr/CBr2 This rate equation has no order of the reaction. n P A n A C A A k P dt dP k C dt dC - = , - = kp=kC (RT)1-n or kC=kp (RT)n-1 The rate law in terms of partial pressures PDF 文件使用 "pdfFactory Pro" 试用版本创建 fwww.fineprint.com.cn f

To sum-up Rate of reaction dCg/vedt Vala=Vglb=Vill=Vylm kala=kg/b=ku/l=kylm 2.Elementary reaction and molecularity 3.For the elementary reaction:aA+bB=IL+mM -dCaldt-kCCgb---rate law dCA=kcC- dPa=k,P呀 dt dt kp=kc(RT)1-n or kc=Kp(RT)n-1 PDF文件使用"pdfFactory Pro”试用版本创建fm,fineprint.com,cn
To sum-up Rate of reaction = dCB 1. /nBdt VA /a=VB /b=VL /l=VM/m kA /a=kB /b=kL /l=kM/m 2. Elementary reaction and molecularity 3. For the elementary reaction: aA+bB=lL+mM -dCA /dt=kCA aCB b ---rate law 4. n P A n A C A A k P dt dP k C dt dC - = , - = kp=kC (RT)1-n or kC=kp (RT)n-1 PDF 文件使用 "pdfFactory Pro" 试用版本创建 Ìwww.fineprint.com.cn f