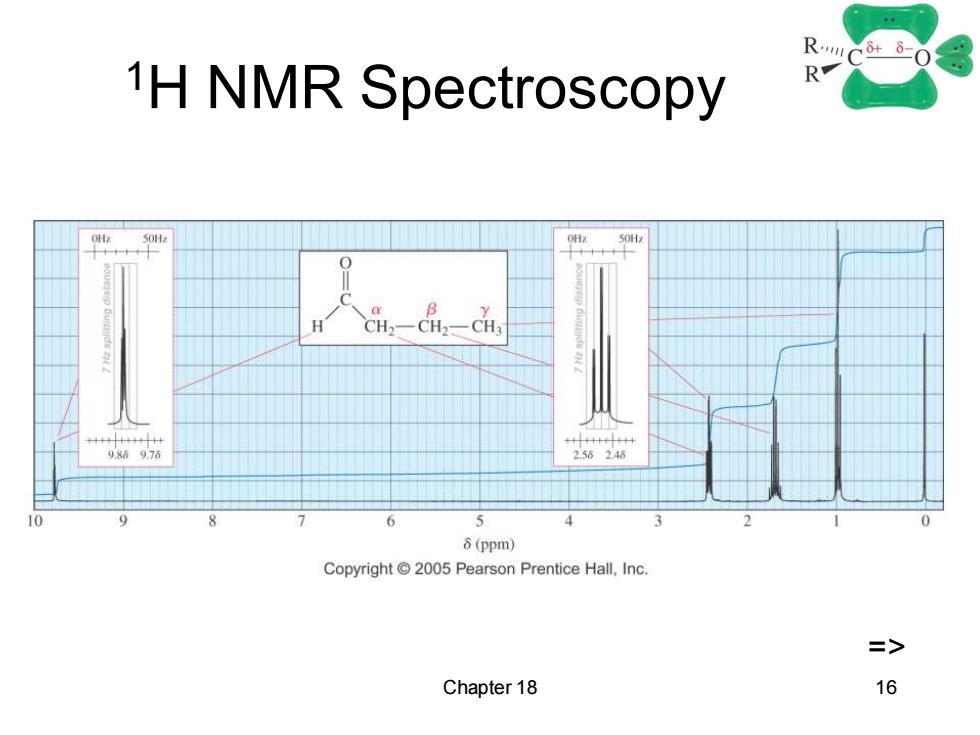
R0 1H NMR Spectroscopy R 9.869.76 256246 10 6 5 8(ppm) Copyright 2005 Pearson Prentice Hall,Inc. => Chapter 18 16
Chapter 18 16 1H NMR Spectroscopy =>
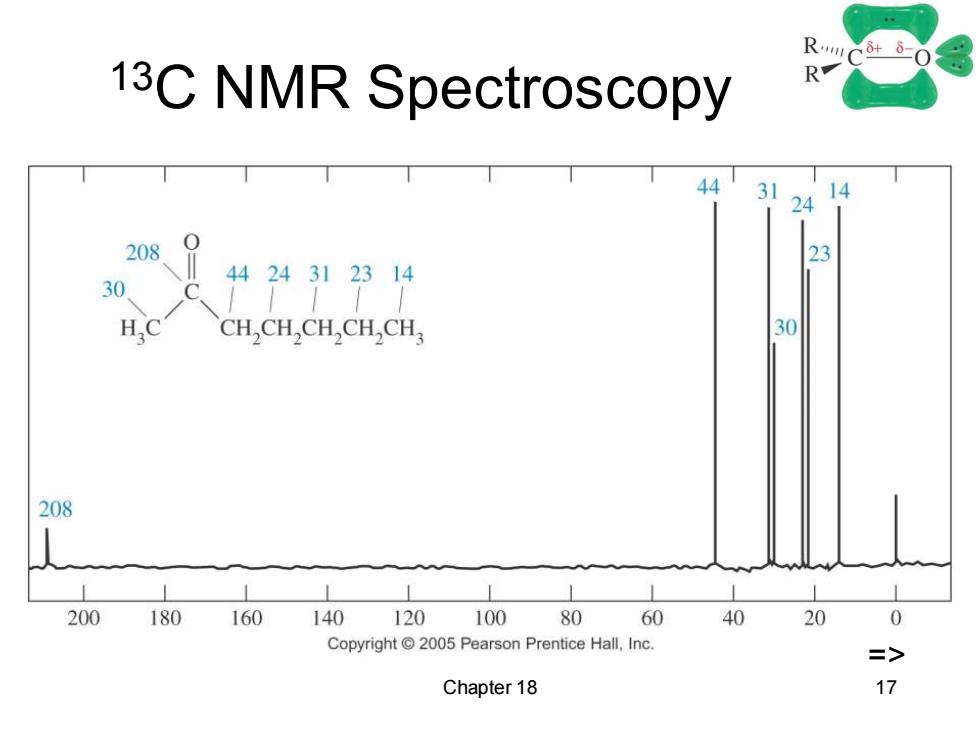
13C NMR Spectroscopy R 44 31 14 24 208、1 23 4424312314 30 C HC CH,CH,CH,CH,CH, 30 208 200 180 160 140 120 100 80 60 40 20 0 Copyright2005 Pearson Prentice Hall,Inc. => Chapter 18 17
Chapter 18 17 13C NMR Spectroscopy =>
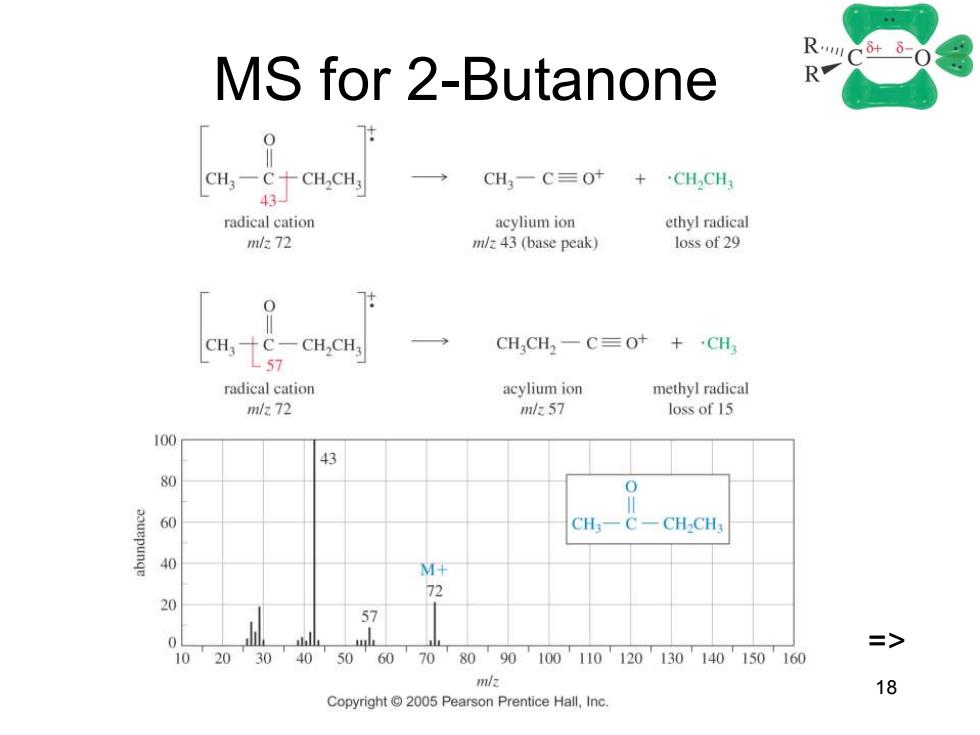
MS for 2-Butanone RC-0 R CH,-C十CH,CH CH3一C≡Ot + ·CH,CH 43☐ radical cation acylium ion ethyl radical m/272 m/z 43(base peak) loss of 29 CH2CH3 CH,CH2一C=Ot+CH radical cation acylium ion methyl radical m/272 m/e57 loss of 15 100 43 80 60 CH一C CH-CH3 M+ 72 20 5> 0 1020 30 40 50 60 70 80 90100110120130140150160 m/2 18 Copyright 2005 Pearson Prentice Hall,Inc
Chapter 18 18 MS for 2-Butanone =>
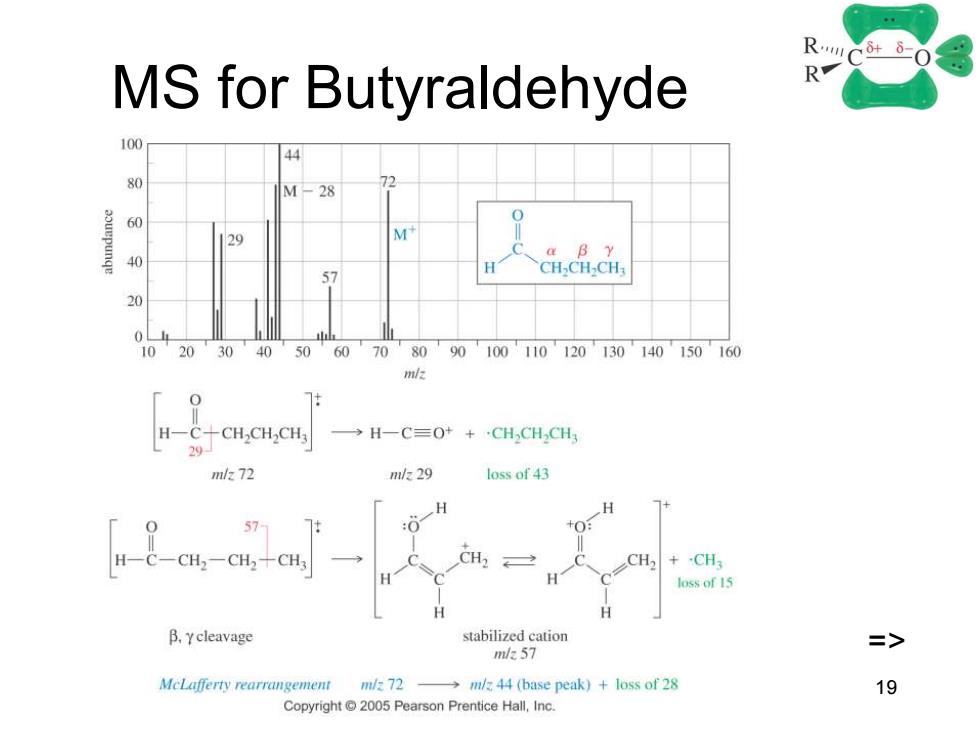
RC-03 MS for Butyraldehyde 100 70 M-28 60 0 29 M B Y % 57 CHCH.CH 10 20 30 40 50 60 70 8090100110120130140150160 m H一CCH,CHCH →H一C=O++,CH,CH CH m/72 n1/k29 loss of 43 H 7+ :0 H一C-CH2-CH2+CH3 CH CH: loss of 15 B.Ycleavage stabilized cation => m/57 McLafferty rearrangement mlz 72 -mlz 44 (base peak)loss of 28 19 Copyright 2005 Pearson Prentice Hall,Inc
Chapter 18 19 MS for Butyraldehyde =>