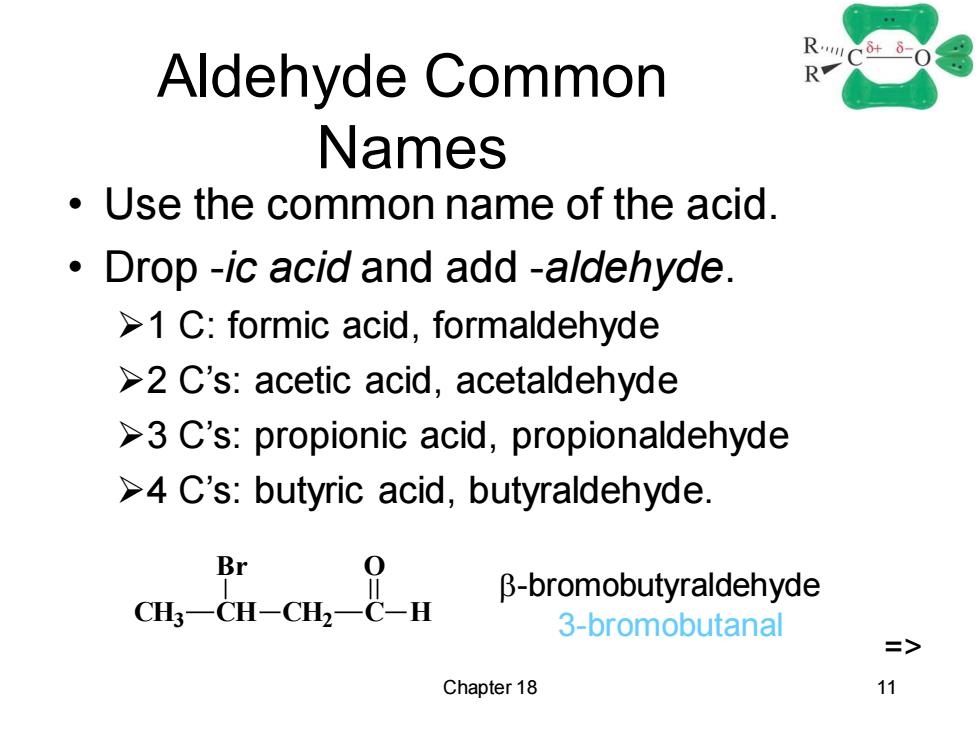
Aldehyde Common RC-0 R Names Use the common name of the acid. Drop -ic acid and add -aldehyde. >1 C:formic acid,formaldehyde >2 C's:acetic acid,acetaldehyde >3 C's:propionic acid,propionaldehyde >4 C's:butyric acid,butyraldehyde. Br B-bromobutyraldehyde 3-bromobutanal => Chapter 18 11
Chapter 18 11 Aldehyde Common Names • Use the common name of the acid. • Drop -ic acid and add -aldehyde. ➢1 C: formic acid, formaldehyde ➢2 C’s: acetic acid, acetaldehyde ➢3 C’s: propionic acid, propionaldehyde ➢4 C’s: butyric acid, butyraldehyde. CH3 CH Br CH2 C H O -bromobutyraldehyde 3-bromobutanal =>
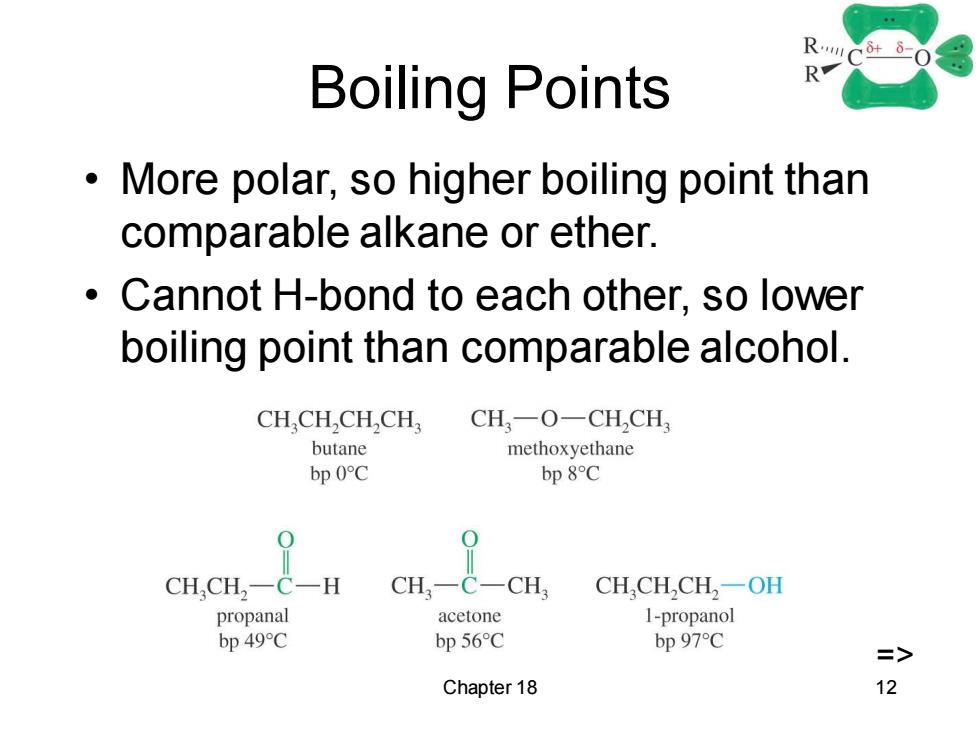
RC-0 Boiling Points R More polar,so higher boiling point than comparable alkane or ether. Cannot H-bond to each other,so lower boiling point than comparable alcohol. CH.CH,CH,CH CH-O一CH,CH butane methoxyethane bp 0C bp 8C CH,CH,-C一H CH一C-CH CH,CH,CH,-OH propanal acetone 1-propanol bp 49C bp 56C bp 97C Chapter 18 12
Chapter 18 12 Boiling Points • More polar, so higher boiling point than comparable alkane or ether. • Cannot H-bond to each other, so lower boiling point than comparable alcohol. =>

R Solubility Good solvent for alcohols. Lone pair of electrons on oxygen of carbonyl can accept a hydrogen bond from O-H or N-H. Acetone and acetaldehyde are miscible in water. => Chapter 18 13
Chapter 18 13 Solubility • Good solvent for alcohols. • Lone pair of electrons on oxygen of carbonyl can accept a hydrogen bond from O-H or N-H. • Acetone and acetaldehyde are miscible in water. =>

RC-0 Formaldehyde Gas at room temperature. Formalin is a 40%aqueous solution. HO OH H 0 H一C H formaldehyde, b.p.-21C formalin trioxane,m.p.62C > Chapter 18 14
Chapter 18 14 Formaldehyde • Gas at room temperature. • Formalin is a 40% aqueous solution. O C O C O C H H H H H H heat H C O H H2O H C H OH HO trioxane, m.p. 62C formaldehyde, b.p. -21C formalin =>
R-0 IR Spectroscopy Very strong C=O stretch around 1710 cm-1. Conjugation lowers frequency. Ring strain raises frequency. Additional C-H stretch for aldehyde:two absorptions at 2710 cm-1 and 2810 cm-1. => Chapter 18 15
Chapter 18 15 IR Spectroscopy • Very strong C=O stretch around 1710 cm-1 . • Conjugation lowers frequency. • Ring strain raises frequency. • Additional C-H stretch for aldehyde: two absorptions at 2710 cm-1 and 2810 cm-1 . =>