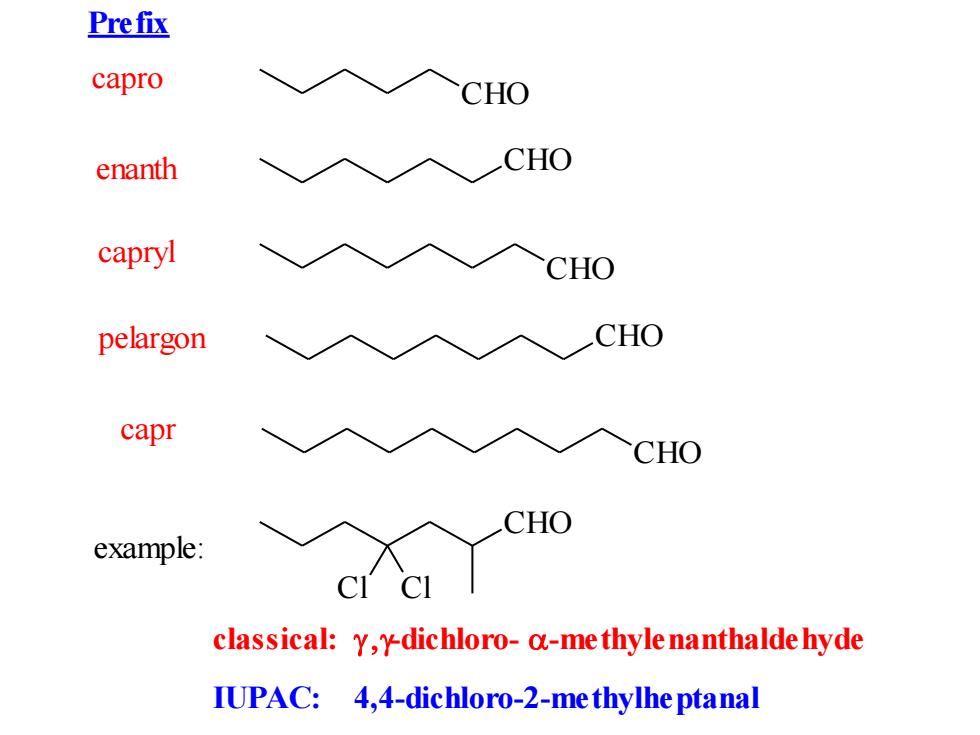
Prefix capro CHO enanth CHO capryl CHO pelargon CHO capr CHO CHO example: classical:y,y-dichloro-a-me thyle nanthalde hyde IUPAC:4,4-dichloro-2-me thylhe ptanal
IUPAC: 4,4-dichloro-2-methylheptanal CHO CHO CHO capro enanth capryl pelargon CHO CHO capr example: CHO Cl Cl classical: -dichloro- -methylenanthaldehyde Prefix
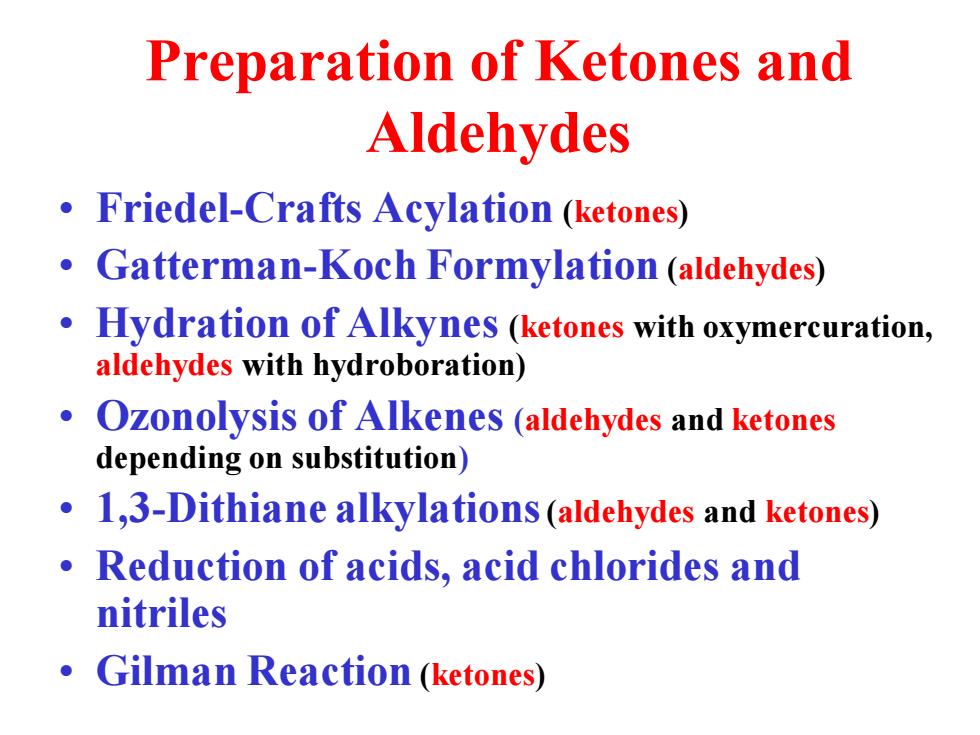
Preparation of Ketones and Aldehydes Friedel-Crafts Acylation (ketones) Gatterman-Koch Formylation (aldehydes) Hydration of Alkynes (ketones with oxymercuration, aldehydes with hydroboration) Ozonolysis of Alkenes (aldehydes and ketones depending on substitution) 1,3-Dithiane alkylations (aldehydes and ketones) Reduction of acids,acid chlorides and nitriles Gilman Reaction (ketones)
Preparation of Ketones and Aldehydes • Friedel-Crafts Acylation (ketones) • Gatterman-Koch Formylation (aldehydes) • Hydration of Alkynes (ketones with oxymercuration, aldehydes with hydroboration) • Ozonolysis of Alkenes (aldehydes and ketones depending on substitution) • 1,3-Dithiane alkylations(aldehydes and ketones) • Reduction of acids, acid chlorides and nitriles • Gilman Reaction (ketones)

Friedel-Crafts Acylation R-C-CI C-R R R is alkyl or aryl;G is hydrogen,a halogen,or an activating group. CI AICl3 02N1 0N1 p-nitrobenzoyl chloride p-nitrobenzophenone (90%)
Friedel-Crafts Acylation

Isoflavones Highly Sought After Natural Products Jamaicin CH3 Piscidia erythrina L. CH3 CHO
Isoflavones Highly Sought After Natural Products O CH3 CH3 O O CH3 O O O Jamaicin Piscidia erythrina L

CH3 OH CICCH2. Friedel-Crafts Acylation A Convergent Synthesis of Flavonoids TiCl4 CH2Cl CH3. CH3 OH +HCI no rxn here CH2O Price,W.A.;Schuda,P.F.J.Org.Chem.,1987,52,1972-197
O CH3 CH3 OH + O O ClCCH2 O CH3O TiCl 4 C H2 C l2 O CH3 CH3 OH O O CH3 O O + HC l no rxn here A Convergent Synthesis Friedel-Crafts Acylation of Flavonoids Price, W.A.; Schuda, P.F. J.Org. Chem., 1987, 52, 1972-1979