
Partition of solutes in two phases based on the difference of their relative solubility. Following formula is also true: cS cm K = = k Vm Vs =b k Differently from in GC, K is relational to properties of mobile phases in LC
Partition of solutes in two phases based on the difference of their relative solubility. Following formula is also true: cS cm K = = k Vm Vs =b k Differently from in GC, K is relational to properties of mobile phases in LC

In order to reduce or eliminate loss of stationary liquid, following measures are usually employed: ●Normal phase liquid-liquid chromatography: Stationary liquid is more polar than mobile phase Stationary liquid: hydrophilic(亲水的) Mobile phase: hydrophobic(疏水的) ●Reverse phase liquid-liquid chromatography: Mobile phase is more polar than stationary liquid Stationary liquid: hydrophobic(疏水的) Mobile phase: hydrophilic(亲水的) ●Chemically bonded stationary phase: 将各种不同有机基团(group)通过化学反应键合到硅胶(silica gel) 担体表面的游离羟基(dissociative hydroxyl)上,代替机械涂敷的液体固 定相。 应用广泛,可用于normal-, reverse-, ion-exchange-, ion pair色谱等 技术中。 Wider applications
In order to reduce or eliminate loss of stationary liquid, following measures are usually employed: ●Normal phase liquid-liquid chromatography: Stationary liquid is more polar than mobile phase Stationary liquid: hydrophilic(亲水的) Mobile phase: hydrophobic(疏水的) ●Reverse phase liquid-liquid chromatography: Mobile phase is more polar than stationary liquid Stationary liquid: hydrophobic(疏水的) Mobile phase: hydrophilic(亲水的) ●Chemically bonded stationary phase: 将各种不同有机基团(group)通过化学反应键合到硅胶(silica gel) 担体表面的游离羟基(dissociative hydroxyl)上,代替机械涂敷的液体固 定相。 应用广泛,可用于normal-, reverse-, ion-exchange-, ion pair色谱等 技术中。 Wider applications

back
back
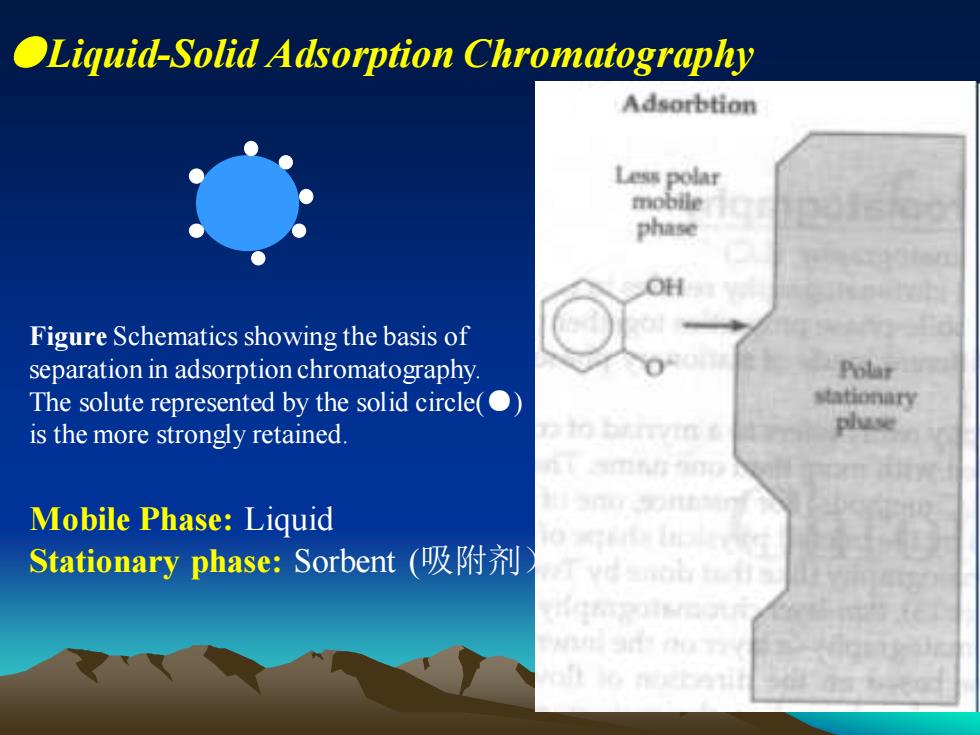
●Liquid-Solid Adsorption Chromatography Figure Schematics showing the basis of separation in adsorption chromatography. The solute represented by the solid circle(●) is the more strongly retained. Mobile Phase: Liquid Stationary phase: Sorbent (吸附剂)
●Liquid-Solid Adsorption Chromatography Figure Schematics showing the basis of separation in adsorption chromatography. The solute represented by the solid circle(●) is the more strongly retained. Mobile Phase: Liquid Stationary phase: Sorbent (吸附剂)

▲Mechanism of liquid-solid adsorption chromatography Separation based on difference of adsorptive actions of substances Competitive adsorption of solute molecule Xand solvent molecule S on active surface of sorbent (溶质分子与溶剂分子在吸附剂活性表 面的竞争吸附): Xm + nSa Xa + nSm where subscripts m and a respectively stand for mobile phase and adsorption phase When adsorption reaches a equilibrium, following formula is true: K = [ Xa ][ Sm] n [Xm][Sa ] n Where K denotes adsorption equilibrium coefficient or partition coefficient
▲Mechanism of liquid-solid adsorption chromatography Separation based on difference of adsorptive actions of substances Competitive adsorption of solute molecule Xand solvent molecule S on active surface of sorbent (溶质分子与溶剂分子在吸附剂活性表 面的竞争吸附): Xm + nSa Xa + nSm where subscripts m and a respectively stand for mobile phase and adsorption phase When adsorption reaches a equilibrium, following formula is true: K = [ Xa ][ Sm] n [Xm][Sa ] n Where K denotes adsorption equilibrium coefficient or partition coefficient