
and Bonding Inductive efTects and carboxylic acids FcH:co pK. 、 Mesomeric effects can also stabilise positive and negative charges 6=8-d-0-8

12 Keynotes in Organic Chemistry as the e lon.as tne o the ter the ilising efec The NO electro-wihdrawin:-and-M 6.c" 9. 1.7.2 Bases In water basicity constant

Structure and Bonding 15 of e deeed y theandal +,0 BHI As HO is in excess · .IfB is Inductive effects and aliphatic (or alkyl)amines R一+CX R-RHX The 。 3 107 N (R=+group)
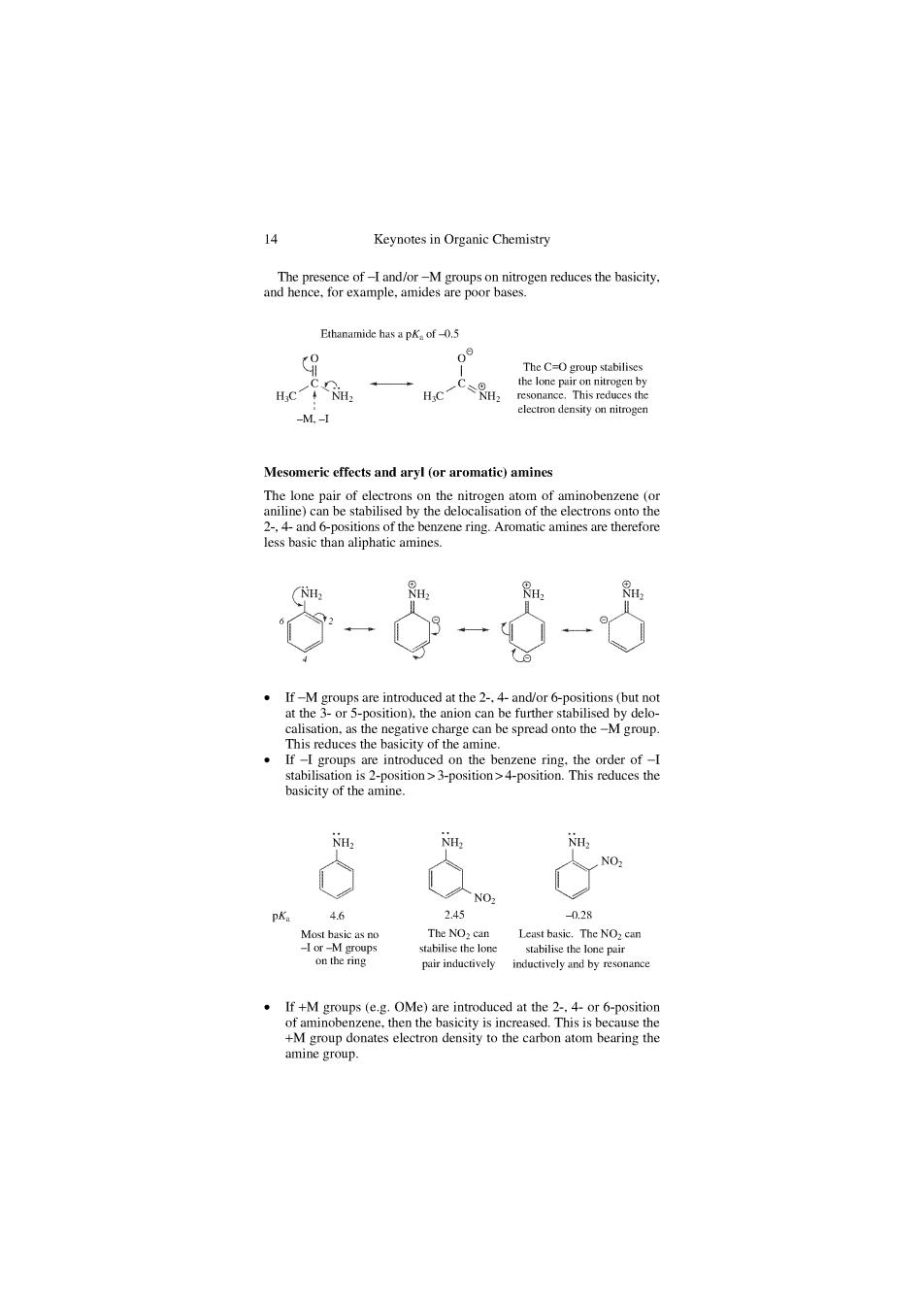
Keynotes in Organic Chemistr nrepT2eoieihdRsnenetacestietesicin 8-6-65 13g puir inductively If+M goups (OMe)re at the6-positior +M amine group
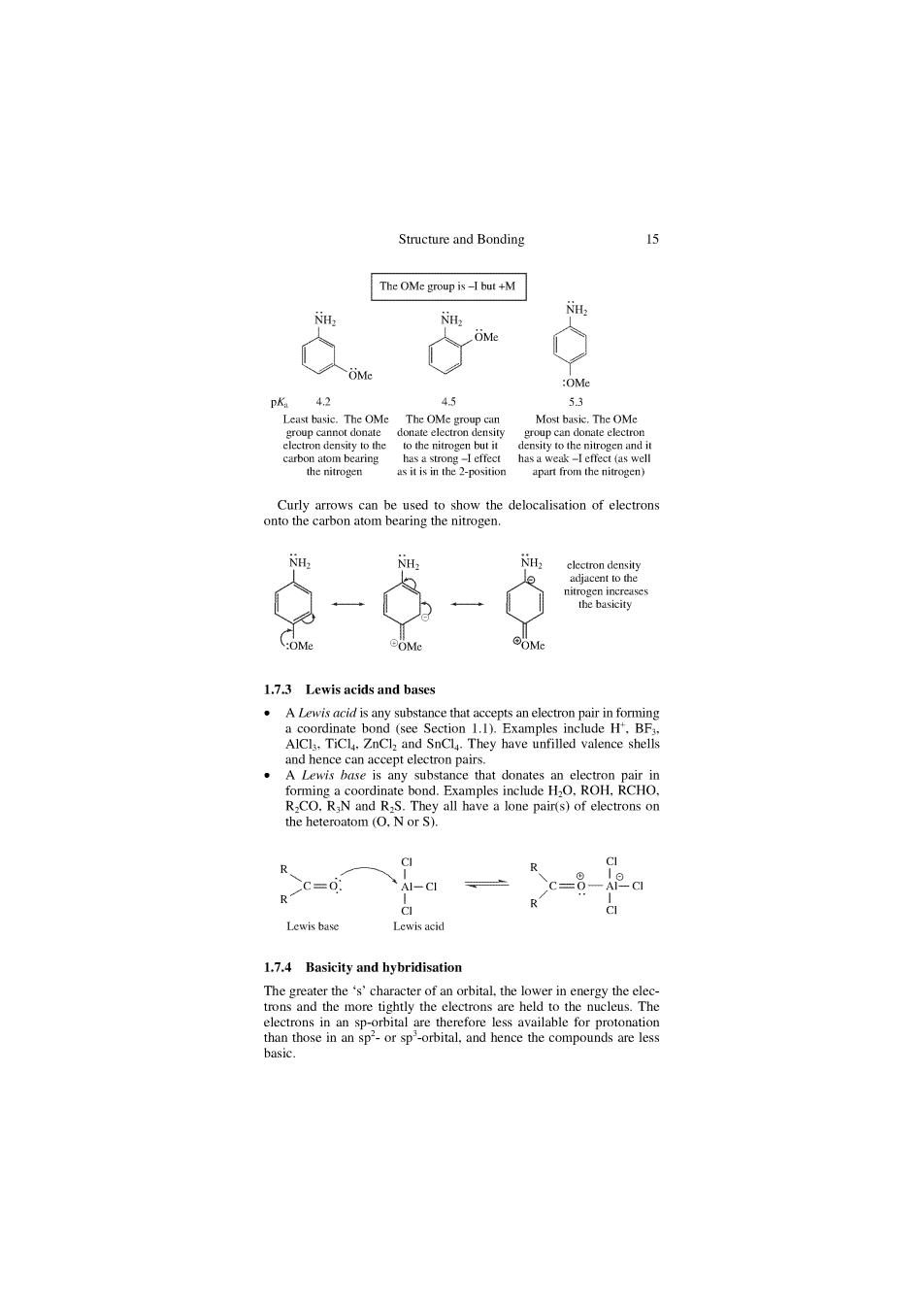
Bonding 15 The OMe group is-1 but +M & pK,42 Most baskc.The OMe - 6-6 1.7.3 Lewis acids and bases .A Lewis acid is any substance that ac Lewis base 1.7.4 Basicity and hybridisation 零 more tightly the held t