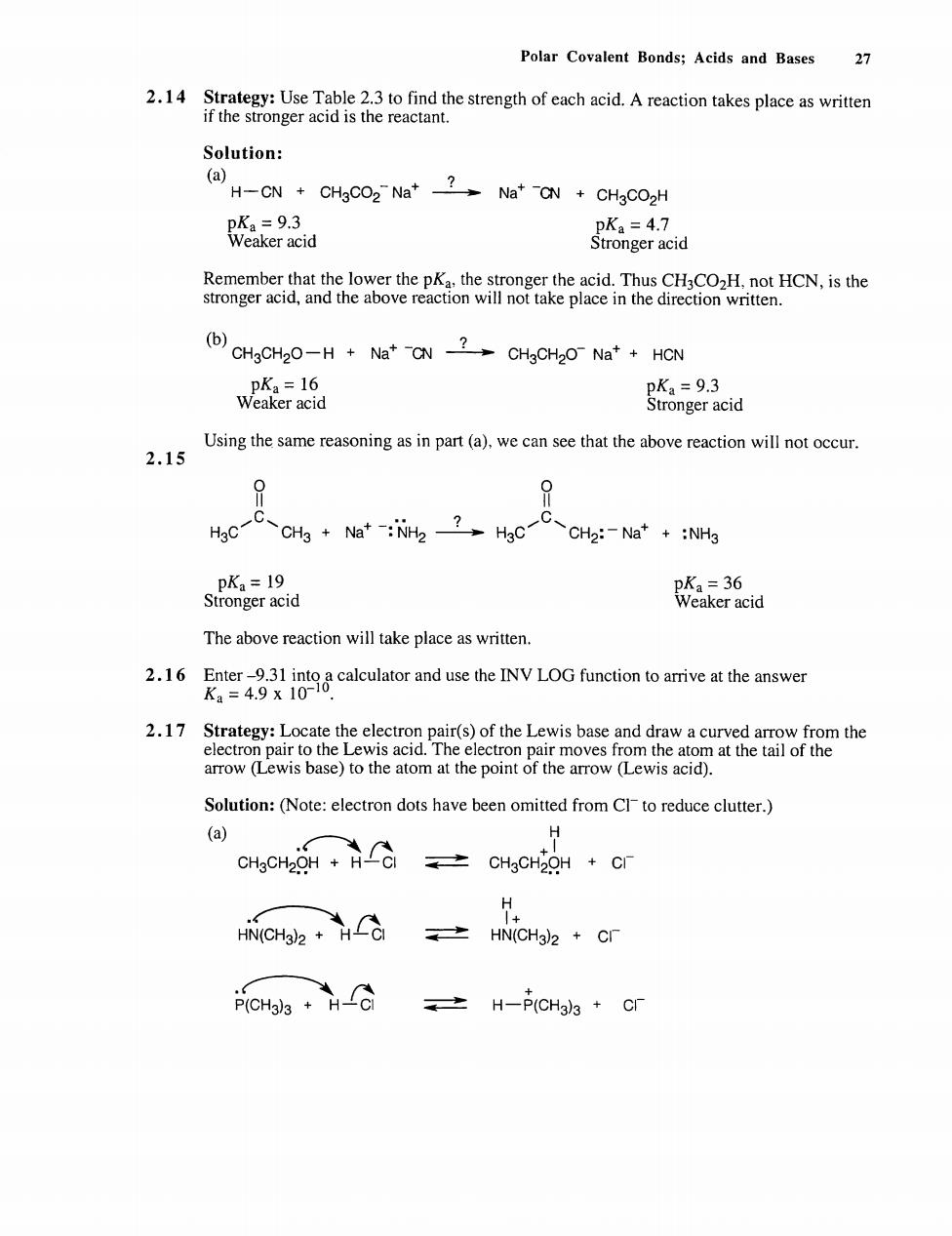
Polar Covalent Bonds;Acids and Bases 27 2.14 Strate Solution: (a) e品d =4.7 emegeieoastekaer8EaeateiCSOaeclHcNstc stronger acid,and the above reaction v (CHaCHaO-H+Na*CNCHaCHO Na+HCN Weater Swonger cid Using the same reasoning as in part(a),we can see that the above reaction will not occur 2.15 P H3c一C、CH+a:Nh22,HgCCH2:-Ne+:NHg eid e36 aker acid The above reaction will take place as written. Solution:(Note:electron dots have been omitted from Clto reduce clutter.) on0 nom.士c40ra·c H HiN(CH)2 HViCH3)2 cr PICHasH 之H-P(CH33+C
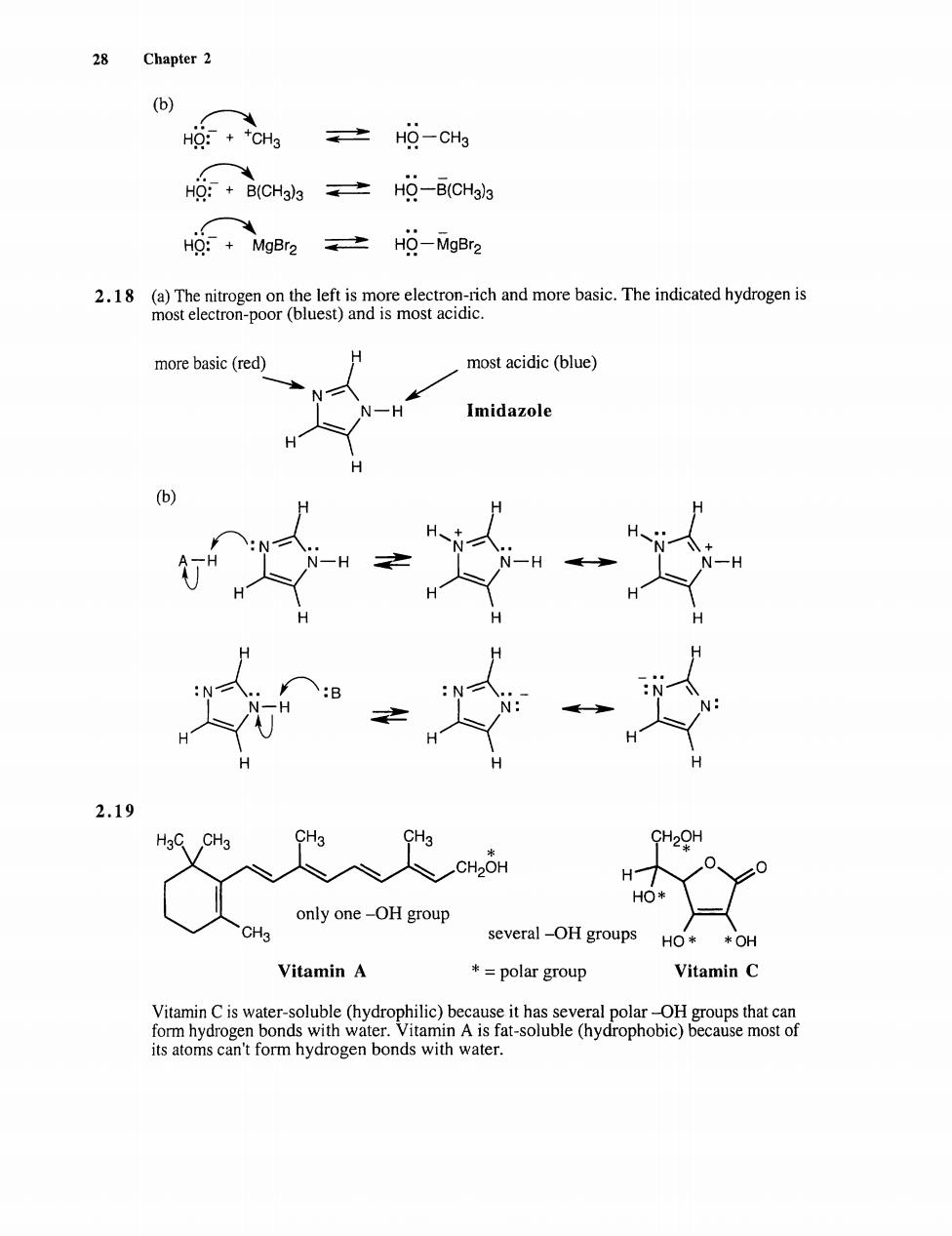
28 Chapter 2 (b) g:+CHa 之HQ-CH3 CH士H9-5Gg Hg+Mgr2。之H9-MgBr2 2.18 (a)The nitrogen on the left is more electron-rich and more basic.The indicated hydrogen is most electron-poor (bluest)and is most acidic. more basic(red) most acidic(blue) Imidazole N 2.19 CH2OH HO* only one-OH group CH3 several-OH groups HO* *OH Vitamin A +=polar group Vitamin C o onbe omo its atoms can't form hydrogen bonds with water
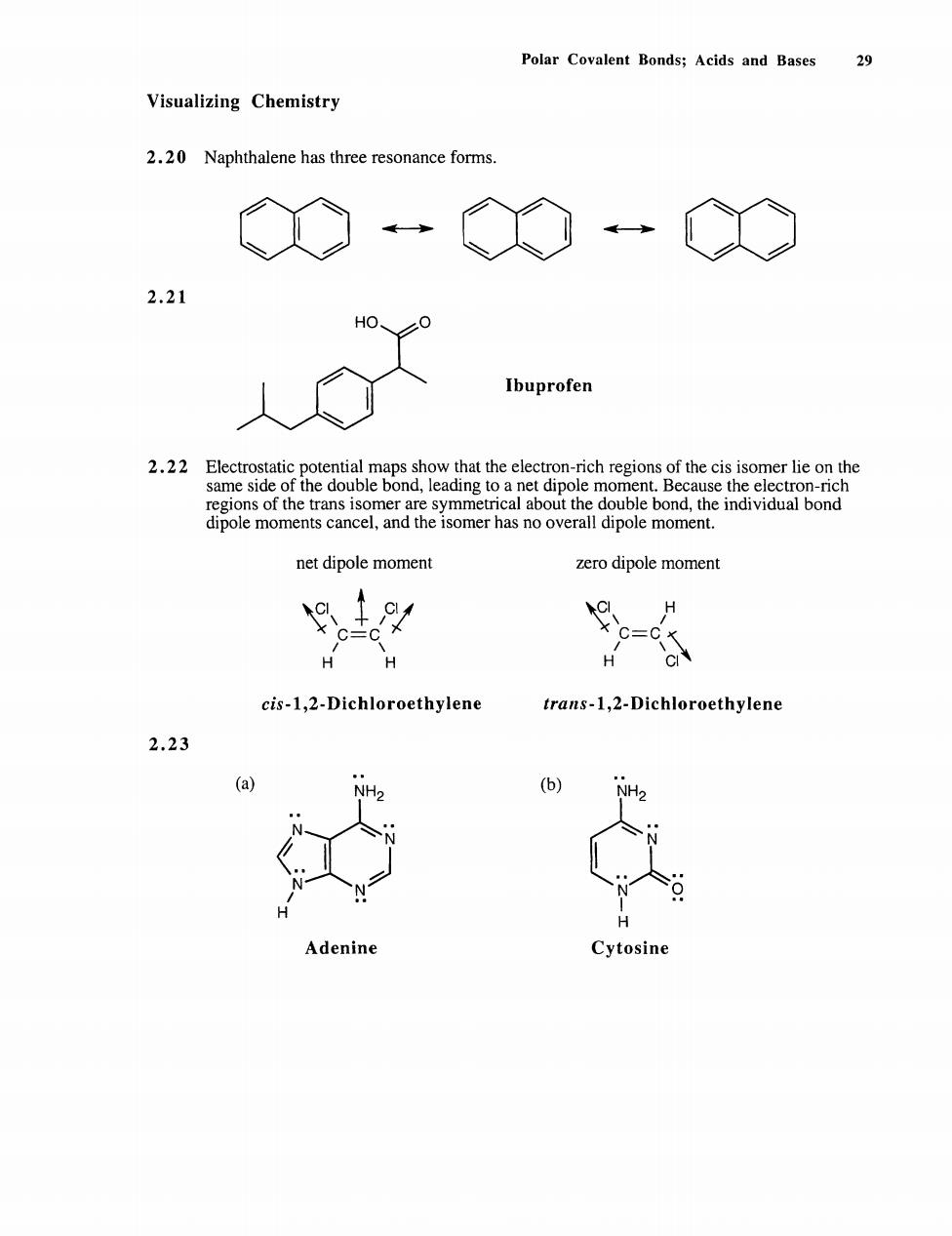
Polar Covalent Bonds;Acids and Bases Visualizing Chemistry 2.20 Naphthalene has three resonance forms. ◇O 一 2.21 人◇ Ibuprofen regonofans isomrareymmetncal aou the doub bond.the indv bond dipole moments cancel,and the isomer has no overall dipole moment. net dipole moment zero dipole moment 9 HH cis-1,2-Dichloroethylene trans-1,2-Dichloroethylene 2.23 (a) (b) .0 Adenine Cytosine

30 Chapter 2 Additional Problems 2.24 773 OH NHCH3 3H Cocaine #=0 hydrogens Ephedrine *1 hydrogen C1H17NO 2.25 Use Figure 2.2 if you nced help.The most electronegative element is starred. (a)CH2FCI (b)FCH2CH2CH2Br (c)HOCH2CH2NH2 (d)CHaOCH2Li 2.26 More polar Less polar a)Hg g 09 (cHsl6s广gg (d)LiOH H 2.27 (a) (b) 2.28 (a)In Section 2.2.we found that =xr.For a proton and an electron separated by 100 m.4.D.If the two charges are separated by 13 pm.6.53 D
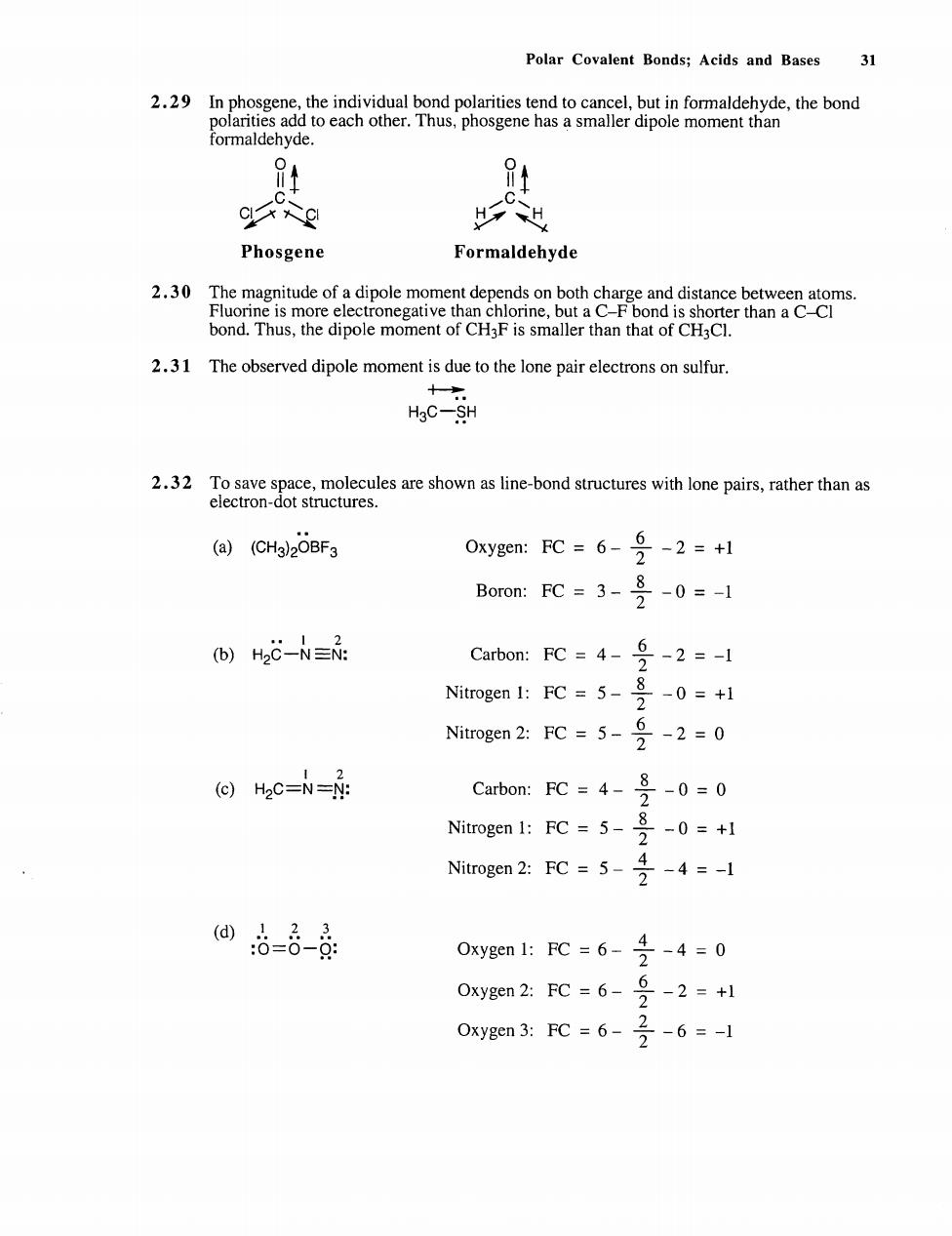
Polar Covalent Bonds;Acids and Bases 31 hemocem mome the ond Phosgene Formaldehyde ” 2.31 The observed dipole moment is due to the lone pair electrons on sulfur. H0- .edon her than (@)(CHa2OBF3 0xyge:FC=6-9-2=+1 Boron:FC=3-号-0=- 间)H2C-N=品: Cabo:FC=4-9-2=-1 Nitrogen 1:FC=5-0=+1 Nitrogen 2:FC=5-2=0 ⊙Hec=N=N Carbon:FC=4-号-0=0 Nirogn 1::Fc=5-号-0=+1 Nitrogen2:FC=5-号-4=1 网6-8-0 Oxygen 1:FC=6-号-4=0 Oxygen2:FC=6-乡-2=+1 0 xygen3:PC=6-2-6=-1