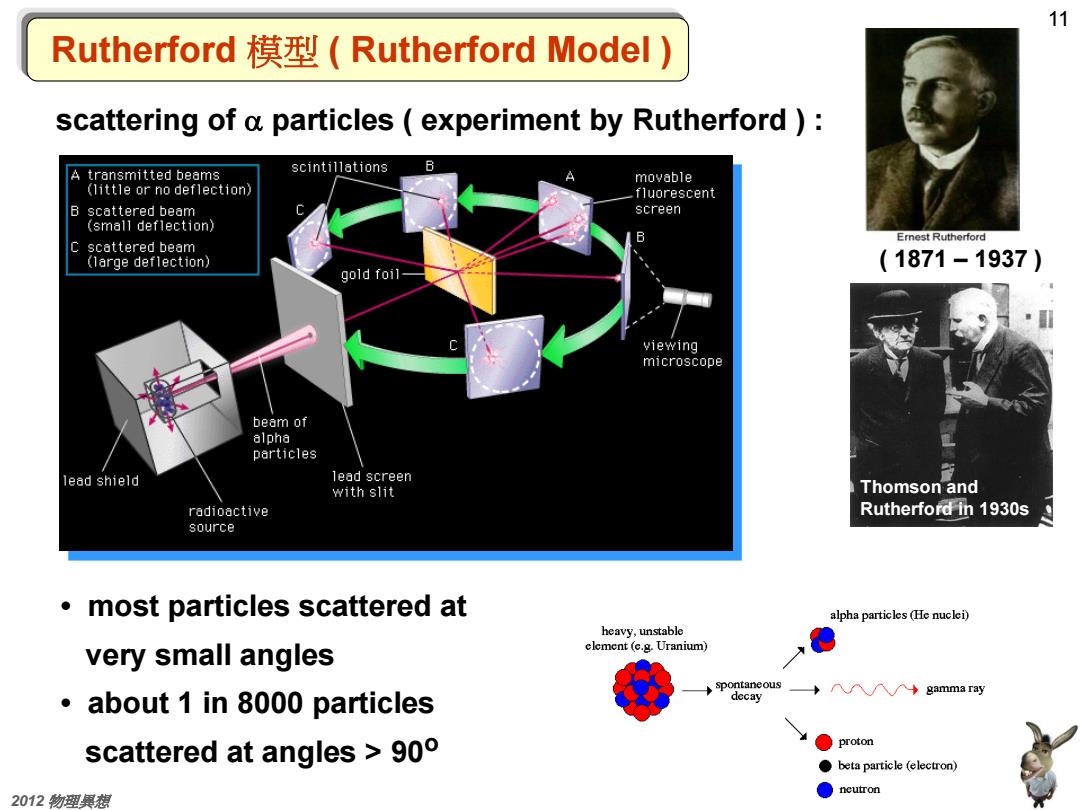
11 Rutherford模型(Rutherford Model) scattering of a particles experiment by Rutherford ) scintillations B transmitted beams A movable (little or no deflection) fluorescent B scattered beam screen (small deflection) Ernest Rutherford C scattered beam (large deflection) (1871-1937) gold foil- viewing microscope beam of alpha particles lead shield lead screen with slit Thomson and radioactive Rutherford in 1930s source most particles scattered at alpha particles (He nuclei) heavy,unstable very small angles element (e.g.Uranium) spontaneous gamma ray about 1 in 8000 particles decay scattered at angles >90 proton beta particle (electron) 2012物理果想 ●ncutron
11 交通大學 李威儀 2012 物理異想 Rutherford 模型 ( Rutherford Model ) scattering of a particles ( experiment by Rutherford ) : • most particles scattered at very small angles • about 1 in 8000 particles scattered at angles > 90o ( 1871 – 1937 ) Thomson and Rutherford in 1930s
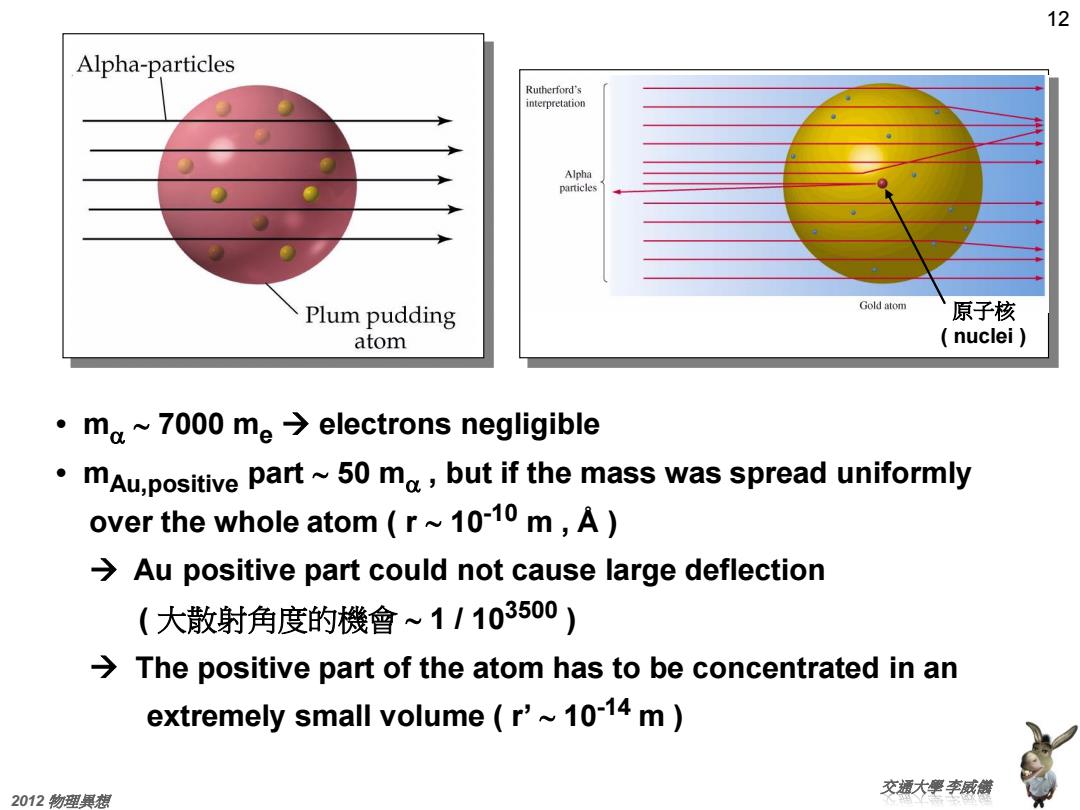
12 Alpha-particles Rutherford's interpretation Alpha particles Plum pudding Gold atom 原子核 atom (nuclei) ·ma~7000me→electrons negligible mAu,positive part~50 ma,but if the mass was spread uniformly over the whole atom (r~10-10 m,A) Au positive part could not cause large deflection (大散射角度的機會~11103500) The positive part of the atom has to be concentrated in an extremely small volume r'~10-14 m 交摄大壁李威截 2012物理興想
12 交通大學 李威儀 2012 物理異想 • ma 7000 me electrons negligible • mAu,positive part 50 ma , but if the mass was spread uniformly over the whole atom ( r 10-10 m , Å ) Au positive part could not cause large deflection ( 大散射角度的機會 1 / 103500 ) The positive part of the atom has to be concentrated in an extremely small volume ( r’ 10-14 m ) 原子核 ( nuclei )