
Propane Conformations: Larger Barrier to Rotation (link) K3ua [enualod 3.3 kcal/mol (14 kJ/mol) 0° 60° 120° dihedral angle H 120° HCH3 60°CH3 HCH3 00
Propane Conformations: Larger Barrier to Rotation (link)
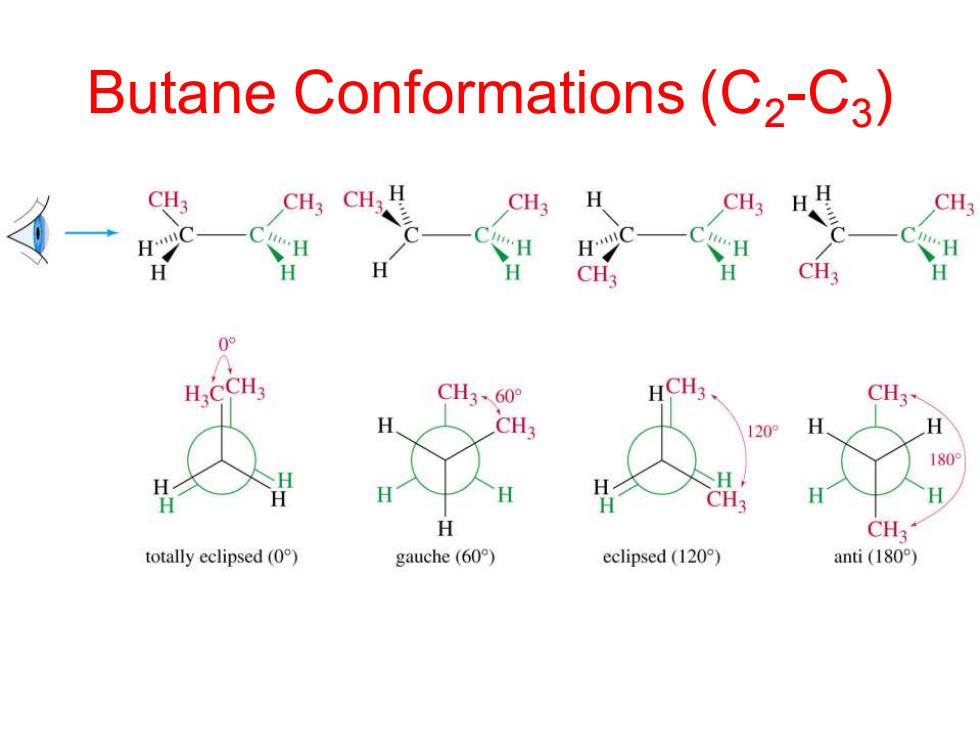
Butane Conformations(C2-C3) CH; CH3 H CH CH3 "H H CH3 H CH3 H 09 H.CCH3 CH3、60° HCH3 CH3 CH3 120° 180 H H CH3 H CH3 totally eclipsed(0) gauche(60) eclipsed(120) anti(180°)
Butane Conformations (C2 -C3 )
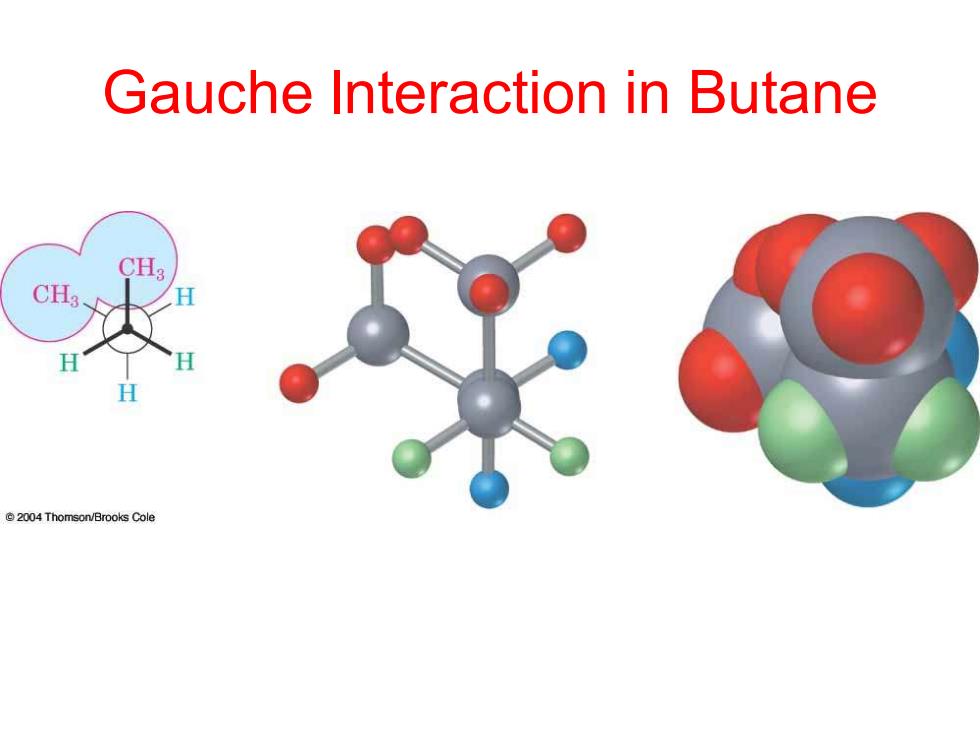
Gauche Interaction in Butane CHa CH3 H @2004 Thomson/Brooks Cole
Gauche Interaction in Butane

2 Different Eclipsed Conformations Cost:6.0 kJ/mol H:CH Total cost:16 kJ/mol H Cost:6.0 kJ/mol Cost:4.0 kJ/mol C2004 Thomson-Brooks/Cole Cost:11 kJ/mol H:CCH3 Total cost:19 kJ/mo Cost:4.0 kJ/mol Cost:4.0 kJ/mol C2004 Thomson-Brooks/Cole
2 Different Eclipsed Conformations

Strain Energy can be Quantified TABLE 4.1 Energy Costs for Interactions in Alkane Conformers Energy cost Interaction Cause (kJ/mol) (kcal/mol) H←→H eclipsed Torsional strain 4.0 1.0 H←→CH2 eclipsed Mostly torsional strain 6.0 1.4 CH3←→CHs eclipsed Torsional plus steric strain 11 2.6 CHg←→CHa gauche Steric strain 3.8 0.9 2004 Thomson-Brooks/Cole
Strain Energy can be Quantified