
1-48 From the amounts of CO2 and H2O generated,the milligrams of C and Hin the original sample can be (a)how much carbon in 14.54 mg CO2 14.54mgC02x 1 mmole CO2 1 mmole C 12.01mgC 44.01mgC02 1 mmole CO2 1 mmole C =3.968mgC how much hydrogen in 3.97 mg H2O 1 mmole H2O 2 mmoles H 1.008mgH 3.97mgH20x 18.016 H 1 mmole H =0.444mgH how much oxygen in 500 mg estradiol 5.00 mg estradiol-3.968 mg C.0.444 mg H 0.59 mg O calculate empirical formula 3.968mgC =0.3304 mmoles C+0.037 mmoles =8.93=9C 12.01 mg/mole 0.444mgH 1.008 mg/mole 0.440 mmoles H 0.037 mmoles 11.9=12 H 0.59mg0 =0.037 mmoles O 0.037 mmoles =10 16.00 mg/mole empirical formula → empirical weight =136 (b)molecular weight =272.exactly twice the empirical weight twice the empirical formula molecular formula C18H24O2
1-48 From the amounts of CO2 and H20 generated, the mi lligrams of C and H in the original sample can be determined, thus giving by difference the amount of oxygen in the 5.00 mg sample. From these values, the empirical formula and empirical weight can be calculated. (a) how much carbon in 14.54 mg CO2 1 mmole CO2 1 mmole C 12.01 mg C 14.54 mg CO2 x x X = 3.968 mg C 44.01 mg CO2 1 mmole CO2 1 mmole C how much h)::drogen in 3.97 mg H2Q 1 mmole H2O 2 mmoles H 1 .008 mg H 3.97 mg H20 x x X = 18.016 mg H2O 1 mmole H2O 1 mmole H 0.444 mg H how much ox)::gen in 5.00 mg estradiol 5.00 mg estradiol - 3.968 mg C - 0.444 mg H = 0.59 mg ° calculate empirical formula 3.968 mg C 12.01 mg/mole 0.444 mg H 1.008 mg/mole 0.59 mg ° 16.00 mg/mole = 0.3304 mmoles C = 0.440 mmoles H = 0.037 mmoles ° empirical formula = � 0.037 mmoles = 8.93 == 9 C 0.037 mmoles = 1 1 .9"" 12 H 0.037 mmoles = 1 ° empirical weight = 136 (b) molecular weight = 272, exactly twice the empirical weight twice the empirical formula = molecular formula = 24
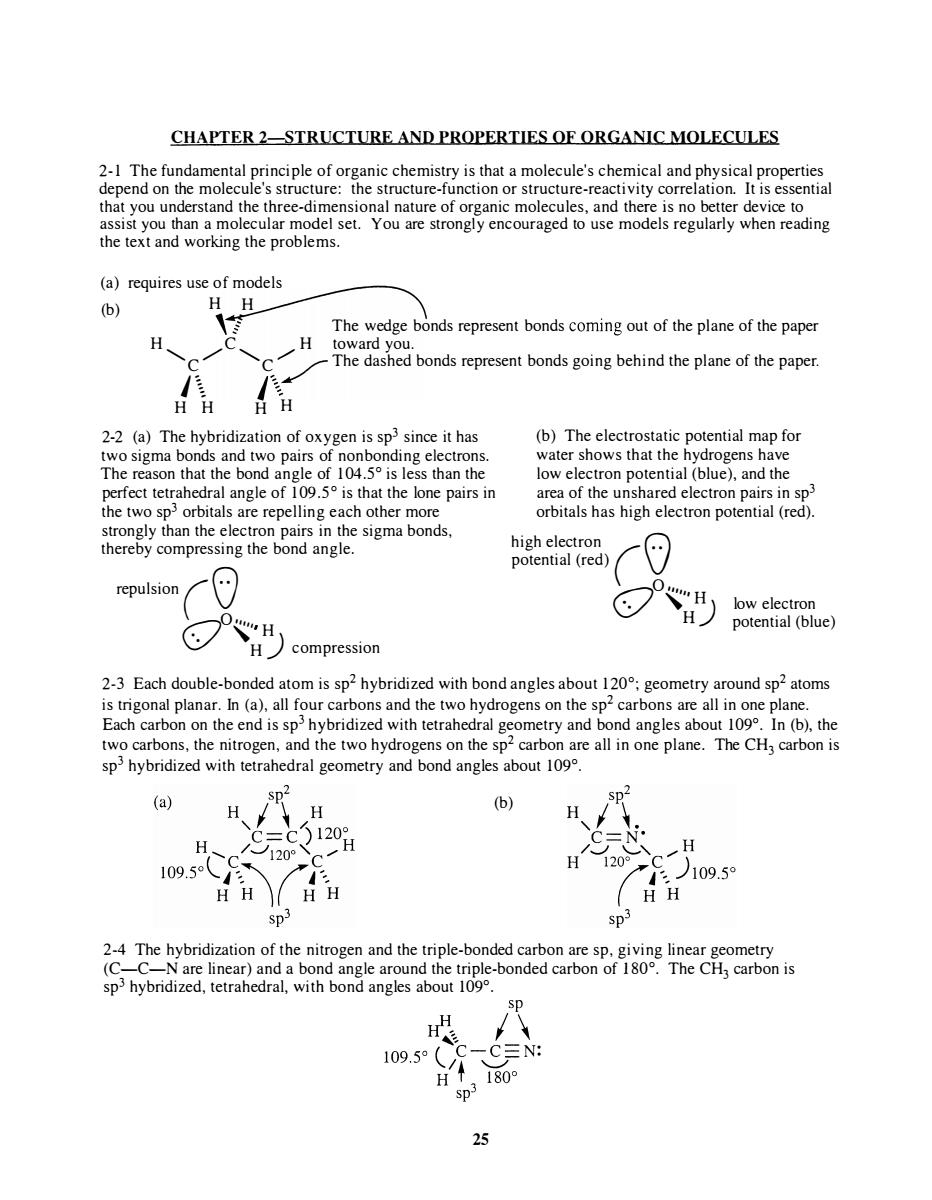
CHAPTER 2-STRUCTURE AND PROPERTIES OF ORGANIC MOLECULES ysical properties (a)requires use of models (6) H The wedge bonds represent bonds coming out of the plane of the paper H H bonds represent bonds going behind the plane of the paper oxygen is sp since it has (b)The electrost tic potential map for pa of 104. esthan the that t th perfect tetrahedral angle of 109.5 is that the lone pairs in area of the unshared electron pairs in sp> the two sp orbitals are repelling each other more orbitals has high electron potential(red). strongly than the electror high electron potential (red) repulsion H potential(blue) H compression 2-3 Each double-bonded atom is sp2 hybridized with bond angles about 120:geometry around sp2 atoms is trigonal planar.In (a),all four carbons and the two hydrogens on the sp2 carbons are all in one plane. Each carbon on the end is sp hybridized with tetrahedral geometry and bond angles about 109.In (b),the two carbons,the nitrogen,and the two hydrogens on the sp2 carbon are all in one plane.The CH3 carbon is sp hybridized with tetrahedral geometry and bond angles about 109. (a) H (b) H 120H 20° 109.5o H H 109.5 sp3 SD 2-4 The hybridization of the nitrogen and the triple-bonded carbon are sp.giving linear geometry (C-c sphybridized,tetrah H 09.5 -CN: 180° 25
CHAPTER 2-STRUCTURE AND PROPERTIES OF ORGANIC MOLECULES 2- 1 The fundamental principle of organic chemistry is that a molecule's chemical and physical properties depend on the molecule's structure: the structure-function or structure-reactivity correlation. It is essential that you understand the three-dimensional nature of organic molecules, and there is no better device to assist you than a molecular model set. You are strongly encouraged to use models regularly when reading the text and working the problems. (a) requires use of models H H (b) , f The wedge bonds represent bonds coming out of the plane of the paper H C H toward you. "C /" """C /" ./ The dashed bonds represent bonds going behind the plane of the paper. J\ J\./ H H H H 2-2 (a) The hybridization of oxygen is sp 3 since it has two sigma bonds and two pairs of non bonding electrons. The reason that the bond angle of 104.5° is less than the perfect tetrahedral angle of 1 09.5° is that the lone pairs in the two sp 3 orbitals are repelling each other more strongly than the electron pairs in the sigma bonds, thereby compressing the bond angle. repulsion (0 OO""H . H ) compression (b) The electrostatic potential map for water shows that the hydrogens have low electron potential (blue), and the area of the unshared electron pairs in sp 3 orbitals has high electron potential (red). high electron (0' potential (red) O O ,�) low electron potential (blue) 2-3 Each double-bonded atom is sp 2 hybridized with bond angles about 120°; geometry around sp2 atoms is trigonal planar. In (a), all four carbons and the two hydrogens on the sp 2 carbons are all in one plane. Each carbon on the end is sp 3 hybridized with tetrahedral geometry and bond angles about 109°. In (b), the two carbons, the nitrogen, and the two hydrogens on the sp2 carbon are all in one plane. The CH3 carbon is sp 3 hybridized with tetrahedral geometry and bond angles about 109°. (b) 2-4 The hybridization of the nitrogen and the triple-bonded carbon are sp, giving linear geometry (C-C-N are linear) and a bond angle around the triple-bonded carbon of 1 80°. The CH3 carbon is sp 3 hybridized, tetrahedral, with bond angles about 109°. 25
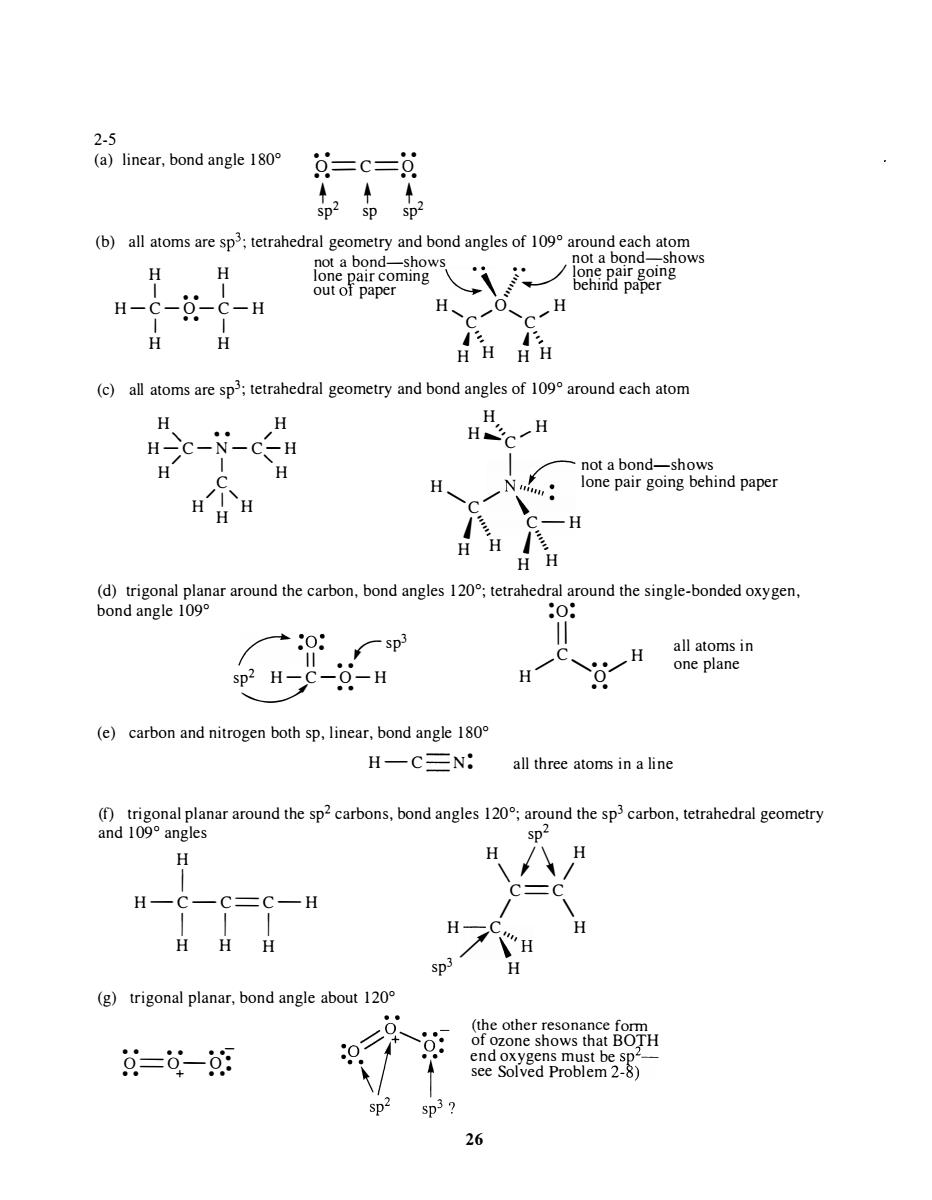
2-5 (a)linear,bond angle180 =c= (b)all atoms are sp3;tetrahedral geometry and bond angles of 109 around each atom H H H-c-0-C-H H H HH HH c) all atoms are sp:tetrahedral geometry and bond angles of 109 around each atom H H总H -H H not a bond- H (d)trigonal planar around the carbon,bond angles 120;tetrahedral around the single-bonded oxygen bond angle 109° :o: +0: sp3 H sp2 H-C-0-H (e)carbon and nitrogen both sp,linear,bond angle 180 H-C三N: all three atoms in a line (f)trigonal planar around the sp2 carbons,bond angles 120;around the sp3carbon,tetrahedral geometry and109°angles H H H -C=C-H H H H (g)trigonal planar,bond angle about 120 (the other reson 文=0-朗 p
2-5 (a) linear, bond angle 1 80° • • • • o==C==o • • • • + + + Sp2 Sp Sp2 (b) all atoms are sp 3 ; tetrahedral geometry and bond angles of 109° around each atom not a bond-shows not a bqnd-;-shows H H lone Rair coming \ • . � � lon� pair gomg I • • I H-C-O-C-H I • • I H H out 01 paper "'--- / behmd paper H , ....... 0, ..... H C C l " , A '" H H H H (c) all atoms are sp 3 ; tetrahedral geometry and bond angles of 109° around each atom H H " . . / H H �' ..... H H-C-N-C-H C / I " H C H I ........-- not a bond-shows H N . lone pair going behind paper / 1 ' H H H '-... C /' " " . J '=:., C-H H " H J\ H H (d) trigonal planar around the carbon, bond angles 120°; tetrahedral around the single-bonded oxygen, bond angle 109° :0: ,.....-- ·0· 3 I .. rsp II •• sp� 2 H-C-O-H .. (e) carbon and nitrogen both sp, linear, bond angle 1 80° H-C N: I I / C " •• / H H ° • • all three atoms in a line all atoms in one plane (0 trigonal planar around the sp2 carbons, bond angles 120°; around the sp 3 carbon, tetrahedral geometry and 109° angles sp2 H I H - C-C==C- H I I I H H H (g) trigonal planar, bond angle about 120° . . . . . .- 0==0-0 · • • + •• - H \ 1\ / H C==C / \ H/C " H \,'H sp 3 H (the other resonance form of ozone shows that BOTH end oxygens must be sp2_ see Solved Problem 2-8) 26
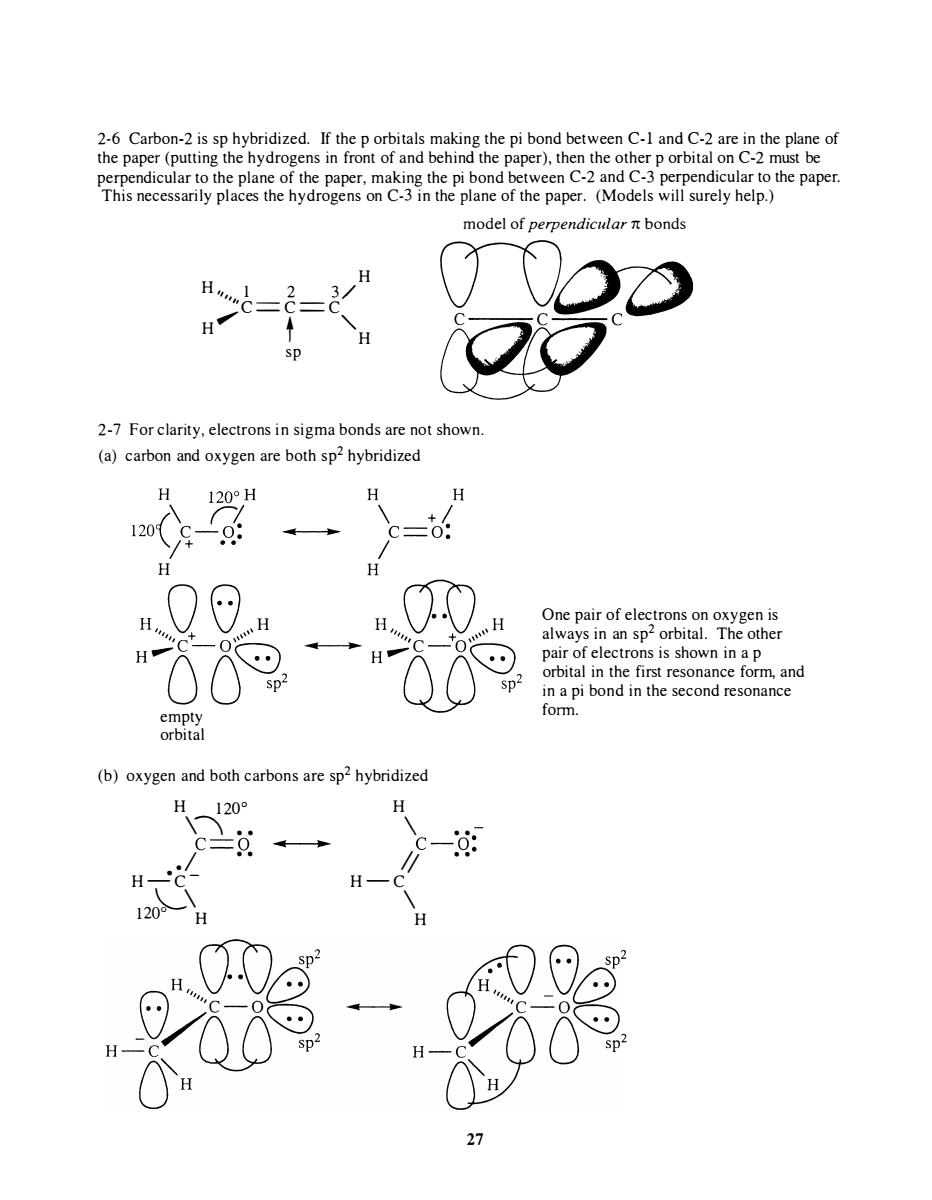
dicular to the pla model of perpendicular n bonds 2-7 For clarity,electrons in sigma bonds are not shown (a)carbon and oxygen are both sp2 hybridized H c-o! 器 One pair of electrons on oxygen is always in an sp2 orbital.The other (b)oxygen and both carbons are sp2 hybridized 120 120
2-6 Carbon-2 is sp hybridized. If the p orbitals making the pi bond between C-l and C-2 are in the plane of the paper (putting the hydrogens in front of and behind the paper), then the other p orbital on C-2 must be perpendicular to the plane of the paper, making the pi bond between C-2 and C-3 perpendicular to the paper. This necessarily places the hydrogens on C-3 in the plane of the paper. (Models will surely help.) model of perpendicular n bonds H H " '" 1 2 3 / 'C ==C==C H' t "' H sp 2-7 For clarity, electrons in sigma bonds are not shown. (a) carbon and oxygen are both sp2 hybridized empty orbital (b) oxygen and both carbons are sp2 hybridized H 1 200 H \\ .. \ .. C==O .... .. I---l .. � C-O: . 1 .. II .. H -·C - H - C 12�\ H \ H 27 One pair of electrons on oxygen is always in an sp2 orbital . The other pair of electrons is shown in a p orbital in the first resonance form, and in a pi bond in the second resonance form
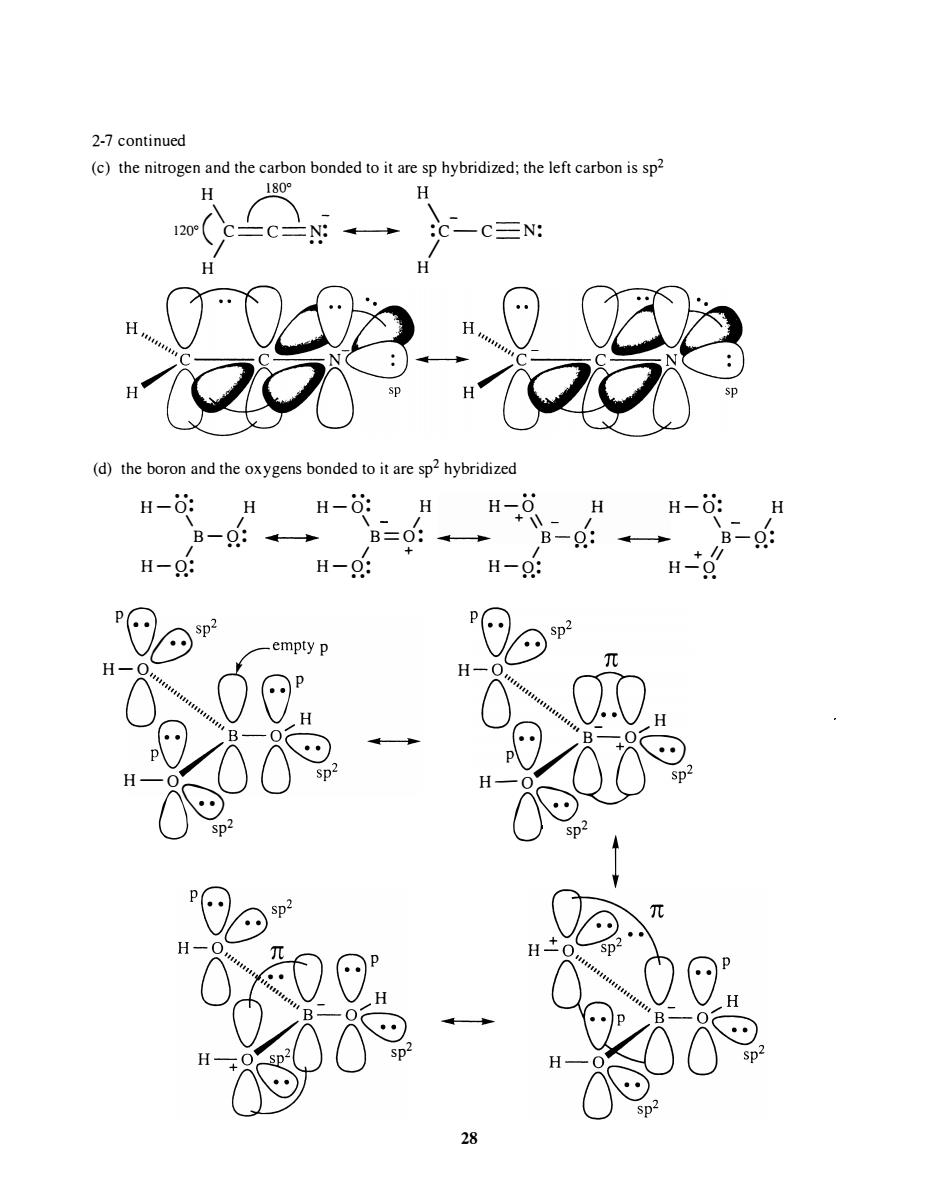
2-7 continued (c)the nitrogen and the carbon bonded to it are sp hybridized:the left carbon is sp 28 (d)the boron and the oxygens bonded to it are sp2 hybridize H-: -6 H-0: H-0
2-7 continued (c) the nitrogen and the carbon bonded to it are sp hybridized; the left carbon is sp2 H 1 800 H 1 200 ( \ c � N: II � �c -- c N: / .. / H H oil (d) the boron and the oxygens bonded to it are sp2 hybridized H-O: H H-O: H H-O H H-O: H \ I \ I + \\ I \ I B-O: II .. B=O: II � B-O: .. � B-O: I •• I + I · • + II •• H-O : H-O : H-O : H-O . . . . . . . . POe SP ' Q(empty p H-O (j" " " "" " '" 0 P PO�o ''B -0 06 - H - O O� sp' sp2 P 2 H - " n " " " " ,:, ' : " (.:) p ' " _ V H H �� � + sp2 •• POes P 2 H {J'Q " " " " '" " " "" ,',\JD _ H .. B-O""'" P VtQD � H - O O� sp ' sp' ! - 28