
1-8 continued H H 6 c=c-N=0 → =c--:→ C-c=N-0: H H: HH:0: HH:0: major and a (c) H-C=g-H→H-c-Q-H H H minor @H-C=N=N:→H--NN: H minor e)H-C-c三N:→H-C=C=N: minor 0H-对--=0--H一→-=-=--H HHHHH HHHHH minor major these two forms are major contributors because all atoms have full octets H-N-c=C-0-N-H--H-N-c=C-C=N-H RRR HH minor major 0:0: 6:0: o:0: gH-c--C-H→H-c=c-c-H→H-C-c=C-H H H H minor major major here aren the more rona ym uivalent en nd are
1-8 continued H H H (b) (c) (d) (e) \ + C=C - N=O / 1 1 H H :0 : • .. \ + C= C-N-O: .. . / 1 11 H H :0 : \ + + C-C=N-O: / 1 1 H H :0 : major major minor These two forms have equivalent energy and are major because they have full octets, more bonds, and less charge separation than the minor contributor. + H-C=O-H .. • H-C- O-H 1 + 1 H H major mmor (octets, more bonds) + + H - C=N=N: .. .- H-C-N N: 1 1 H H major (negative charge minor on electronegative atom) H-C-C N: .. • H - C=C=N: 1 1 H H minor major (negative charge on electronegative atom) + + (f) H-N -C-C=C- N-H " • H-N=C- C=C-N-H (g) 1 1 1 1 1 1 1 1 1 1 H H H H H H H H H H mmor major t these two forms are major contributors because al l atoms have full octets + •• + H-N- C= C- C-N-H .. • H - N-C=C-C=N-H 1 1 1 1 1 1 1 1 1 1 H H H H H H H H H H mmor major .. - :0 : :0 : : 0: :0 : :0 : :0 : II • • II 1 II II 1 H-C-C-C-H----- H-C=C-C-H ----- H-C-C=C-H 1 - 1 1 H H H mITIor major major these two have equivalent energy and are major because the negative charge is on the more electronegative oxygen atom 4
1-8continued 0: :0: (h)H-C-N-H H-C=N-H minor H ef ae atoms on the page is not significant.A Lewis structure is"complete"with unshared electron pairs shown. HHHHHH HHH (a)H-C-c-c_ C-H (b)H-C-C HH HH H HC、H H H HH:0: (C)H-C (d)H-C HH kaSecgomt are narges H:O: HH H (e)H-( -C-C三N: H 0: H (0)H-c-c -c-0-H H HH:O:HH (g)H-C -C-H HH 1-10 Complete Lewis structures show all atoms,bonds,and unshared electron pairs. (a) (b) H (c) H H HH H -H H C-H H -H
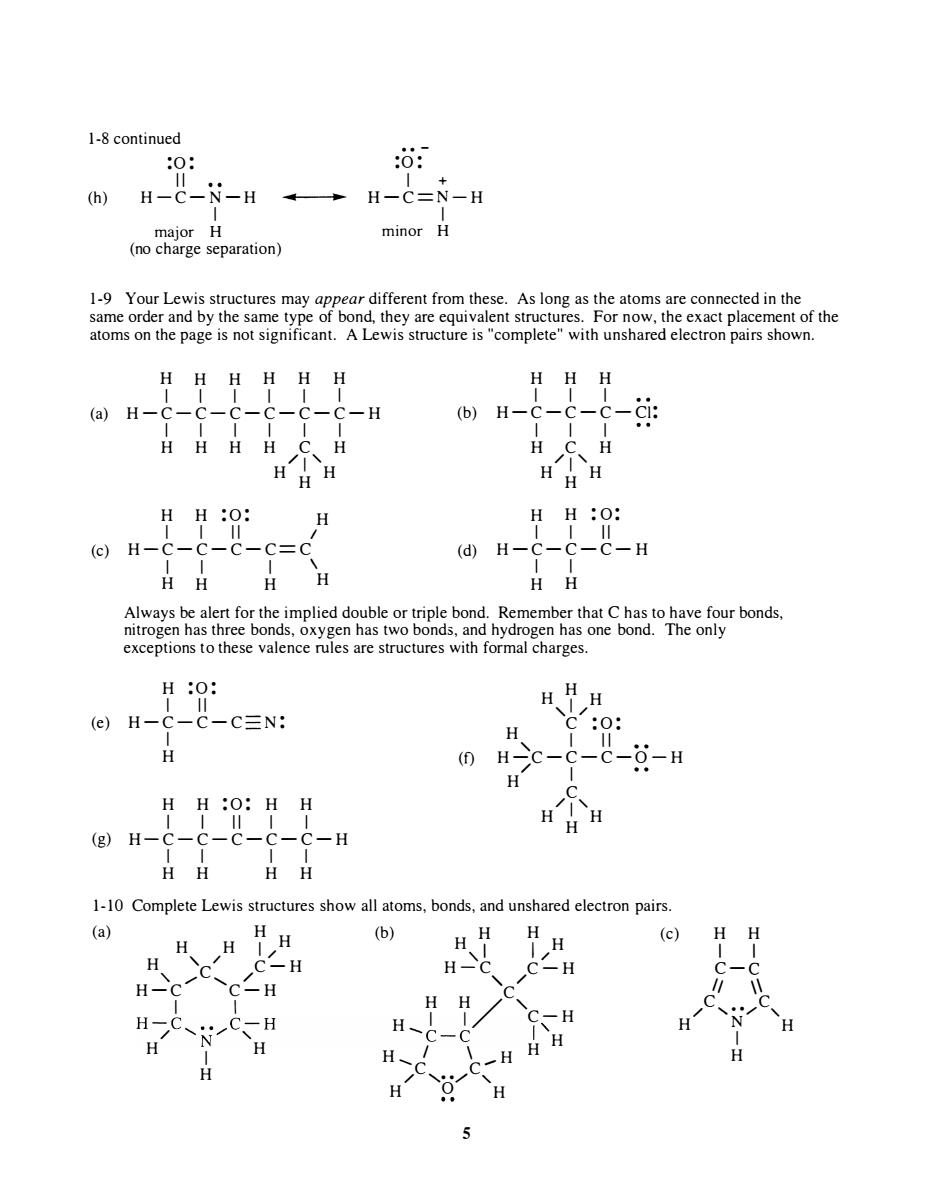
1-8 continued (h) :0 : II H-C -N-H I major H (no charge separation) .. - :0 : I + .... .. ---I .. � H - C = N - H I minor H 1-9 Your Lewis structures may appear different from these. As long as the atoms are connected in the same order and by the same type of bond, they are equivalent structures. For now, the exact placement of the atoms on the page is not significant. A Lewis structure is "complete" with unshared electron pairs shown. (a) (c) H H H H H H H H H I I I I I I I I I H - C-C-C-C-C-C-H (b) H - C-C-C-Cl: I I I I I I I I I H H H H C H H C H / 1 ' H H H / 1 ' H H H H H :0 : H H H :0: I I II / I I II H-C-C-C-C=C (d) H-C -C-C-H I I I \ I I H H H H H H Always be alert for the implied double or triple bond. Remember that C has to have four bonds, nitrogen has three bonds, oxygen has two bonds, and hydrogen has one bond. The only exceptions to these valence rules are structures with formal charges. H :0: I II (e) H - C-C-C N: H H H , 1 / H C :0: , I I II H (f) H-C -C-C-O-H H H :0: H H I I II I I / H I C (g) H-C-C-C-C-C-H / 1 ' H H H 1-10 (a) I I I I H H H H Complete Lewis structures show all atoms, bonds, and unshared electron pairs. H H (b) H H H (c) H H I H , / / ,I 1/ H C C-H H-C C-H , / , / , / H-C C-H H H C , I I H I 1/ C-H H-C� • • �C -H _ I ' / N' , -C -C H H I H H I \ H H ...... C C ...... H / , •• ,....... , H 0 H 5 H H I I C -C II \\ C, . . /C / N ' H H I H
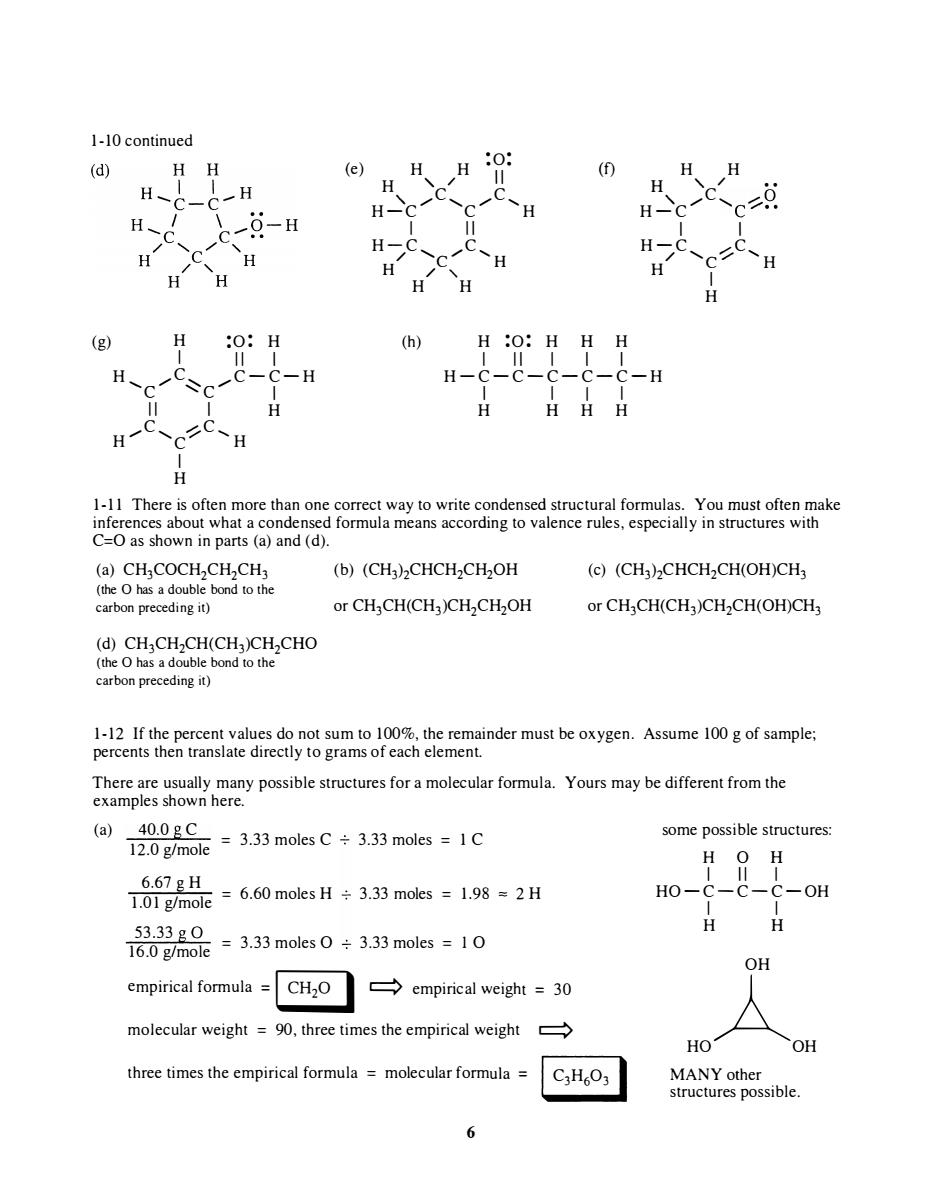
1-10 continued (d) (e) ( H H 0-H H H (g) (h) H :O:HHH H H- C-H C=as shown in parts (a)and (d). (a)CHCOCH,CH,CH (b)(CH3)2CHCH2CH2OH (c)(CH)CHCH2CH(OH)CH (the o has a double bond to the carbon preceding it) or CH3CH(CH3)CH2CH2OH or CH3CH(CH3)CH2CH(OH)CH3 (d)CHCHCH(CH)CH,CHO (the has a double bond to the carbon preceding it) 1-12 If the percent values do not sum to00%.the remainder must be oxygen.Assume 100g of sample; percents then translate directly to grams of each element. There are usually many possible structures for a molecular formula.Yours may be different from the examples shown here. (a) some possible structures 6.67gH 1.01 g/mole ,=6.60 moles H÷3.33 moles=1.98=2H HO- -OH 23品=33m033=10 H H OH empirical formula CH2O empirical weight =30 molecular weight =90,three times the empirical weight HO OH three times the empirical formula=molecular formula= C3H6O3 struct 6
1-10 continued (g) H :0: H I II I H /C:--.., /,C-C-H 'c "c I II I H /C, 'iC, H C H I H ·0· ( ) H H ·· e H ,, / II (f) H H " /C, /C, H-C C H I II H-C, ........ C, / C H H / "- H H (h) H :0: H H H H ,, / •• " /C .... ,...-0 H-C C/·· I I H-C, /.C, / C/' H H I H I II I I I H-C - C-C-C-C-H I I I I H H H H 1-1 1 There is often more than one correct way to write condensed structural formulas. You must often make inferences about what a condensed formula means according to valence rules, especially in structures with C=O as shown in parts (a) and (d). (a) CH3COCH2CH2CH3 (the 0 has a double bond to the carbon preceding it) (d) CH3CH2CH(CH3)CH2CHO (the 0 has a double bond to the c arbon preceding it) (b) (CH3hCHCH2CH20H or CH3CH(CH3)CH2CH20H (c) (CH3hCHCH2CH(OH)CH3 or CH3CH(CH3)CH2CH(OH)CH3 1-12 If the percent values do not sum to 100%, the remainder must be oxygen. Assume 100 g of sample; percents then translate directly to grams of each element. There are usually many possible structures for a molecular formula. Yours may be different from the examples shown here. (a) 40.0 g C = 3.33 moles C 3.33 moles = 1 C 12.0 g/mole 6.67 g H 6.60 moles H 3.33 moles = 1.98 == 2 H 1.01 g/mole = 53.33 gO = 3.33 moles ° 3.33 moles = 1 ° 16.0 g/mole empirical formula = � c::::::> empirical weight = 30 molecular weight = 90, three times the empirical weight c:::::> three times the empirical formula = molecular formula = I C3H603 I 6 some possible structures: H ° H I II I HO -C-C-C- OH I I H H OH HoAOH MANY other structures possible

1-12 continued (b)32.0gC 12.0 g/mole 2.67 moles C+1.34 moles 1.99 =2C some possible structures: HH 6.67gH 1.01 g/mole 6.60 moles H+1.34 moles 4.93 =5 H H-C- C-NO2 18.7gN HH 14.0 g/mole 1.34 moles N+1.34 moles IN H H 42.6g0 T6.0 g/mole =2.66 moles O 1.34 moles =1.9920 -0-C-H empirical formula H C2HsNO2 empirical weight =75 manY other molecular weight =75.same as the empirical weight → structures possible. empirical formula molecular formula (c)37.2gC T2.0 g/mole=3.10 moles C1.55 moles =2C 7.75gH 1.01 g/mole =7.67 moles H 1.55 moles =4.955H 320品c-155mole:a÷l5mes=1d empirical formula=C,HCI empirical weight =64.46 C-q molecular weight=64.same as the empirical weight empirical formula =molecular formula C2HgCl (d) 12.0g/mole =3.20 moles C1.60 moles =2C 38.4pC some possible structures: H H 4.80gH 1.01 g/mole =4.75 moles H1.60 moles =2.97=3H H-C-C=C-C-H H CICI H C CI empirical formula= empirical weight =62.45 molecular weight=125,twice the empirical weight → C twice the empirical formula =molecular formula CaH Cl2 MANY other CI structures possible
1-12 continued (b) 32.0 g C 12.0 g/mole 6.67 g H l.01 g/mole = 2.67 moles C 1.34 moles = 1.99 ::= 2 C 6.60 moles H -:- 1.34 moles = 4.93 5 H IS.7 g N = 1.34 moles N -:- 1.34 moles = 1 N 14.0 g/mole 42.6 g a _ . 16.0 g/mole - 2.66 moles a -;- 1.34 moles = 1 .99 ::= 2 a empirical formula = I C2H sN02 I c:::::::> empirical weight = 75 molecular weight = 75, same as the empirical weight c:::::::> empirical formula = molecular formula = C2HsN02 I (c) 37.2 g C = 3.10 moles C -:- 1 .55 moles = 2 C 12.0 g/mole (d) 7.75 g H = 7.67 moles H -:- 1.55 moles = 4.95 ::= 5 H 1.01 g/mole 55.0 g CI _ . _ 35.45 g/mole - 1 .55 moles Cl -;- 1.55 moles - 1 CI empirical formula = I C2H sCl I c:::::::> empirical weight = 64.46 molecular weight = 64, same as the empirical weight c:::::::> empirical formula = molecular formula = I C2HsCI I 3S.4 g C 12.0 g/mole = 3.20 moles C 1.60 moles 2C 1.� · ��/�:le = 4.75 moles H -:- 1 .60 moles = 2.97 3 H 3��4� ��ole = 1.60 moles CI -:- 1 .60 moles = 1 Cl empirical formula = I C2H 3Cl I c:::::::> empirical weight = 62.45 molecular weight = 1 25, twice the empirical weight c:::::::> twice the empirical formula = molecular formula = C-4-H-C -12---o. , 7 some possible structures: H H I I H-C-C-N02 I I H H H a H \ II I N- C-O-C-H / I H H MANY other structures possible. There is only one structure possible with this molecular formula: H H I I H - C-C-Cl I I H H some possible structures: H H I I H-C- C==C- C- H I I I I H Cl Cl H ClU C] Cl � MANY other Cl structures possi ble

1-13 (a)500 g HBr x I mole H地r =0.0618 moles HB 80.9 g HBr 0.0618 moles HBr 0.0618 molesHO+(100%dissociated) 0.0618 moles H3O* 1000mL 0.618 moles H3O 100mL IL ILsolution pH=-log1oH30*】=-log10(0.618)= 0.209 (b)1.50g NaOH x 1 mole NaOH 0.0375 moles NaOH 40.0g NaOH 0.0375 moles NaOH 之 0.0375 moles-OH (100%dissociated) 0.0375 moles-OH 1000mL 0.75 moles -OH 50.mL 1Lsolution =0.75M (H,0*1=x10 =x10-4 =1.33x10-14 [OH] 0.75 (th nhe of decimal place pH=-log1oH0*】=-log101.33x10-4)= 13.88 in a pH value is the number of significant figures) 11A onia has a proton bonded to must have apairo of share with a proton;in theory,any atom with an unshare is tooeak na tproton:instead,tactsbsnd pullsproton rom tomall extent. (b)water as an acid:H2O NH3OH NH* water as a base: H20+HCI÷HO*+C1 (c)methanol as an acid:CH3OH NH3 CH3O-+NHa" methanol as a base:CH3OH H2SO CH3OHz++HSO 8
1-13 (a) 5.00 g HBr x 1 mole HEr 80.9 g HBr = 0.0618 moles HBr (b) 1-14 0.0618 moles HEr 0.0618 moles H30 + (100% dissociated) 0.0618 moles H30 + 100 mL x 1000 mL 1 L = 0.618 moles H30 + 1 L solution pH = - 10gIO [H30+] = - loglo (0.618) = � 1.50 g NaOH x 1 mole NaOH 40.0 g NaOH = 0.0375 moles NaOH 0.0375 moles NaOH 0.0375 moles -OH (100% dissociated) 0.0375 moles -OH 50. mL 1 x 10- 1 4 [ H 3 0+] = [-OH] x = 1000 mL lL 1 x 10- 1 4 0.75 = 0.75 moles -OH 1 L solution = 1.33 x 10-1 4 pH = -loglO [H30+] = -loglo (1 .33 x 10-1 4 ) = 0.75 M (the number of decimal places in a pH value is the number of significant figures) (a) By definition, an acid is any species that can donate a proton. Ammonia has a proton bonded to nitrogen, so ammonia can be an acid (although a very weak one). A base is a proton acceptor, that is, it must have a pair of electrons to share with a proton; in theory, any atom with an unshared electron pair can be a base. The nitrogen in ammonia has an unshared electron pair so ammonia is basic. In water, ammonia is too weak an acid to give up its proton; instead, it acts as a base and pulls a proton from water to a small extent. (b) water as an acid: H2O + NH3 -- -OH + NH4+ water as a base: H2O + HCl H3O+ + Cl- (c) methanol as an acid: CH30H + NH3 CH3O- + NH + 4 methanol as a base: CH30H + H2SO4 CH3OH2+ + HS04- 8