
The Kinetic Theory of Gas
The Kinetic Theory of Gas

Agenda Today ·Ideal gas Pressure and temperature micro interpretation) Internal energy of idea gas Distribution law of molecular speed for ideal gas mean free path
Agenda Today • Ideal gas • Pressure and temperature ( micro interpretation) • Internal energy of idea gas • Distribution law of molecular speed for ideal gas • mean free path
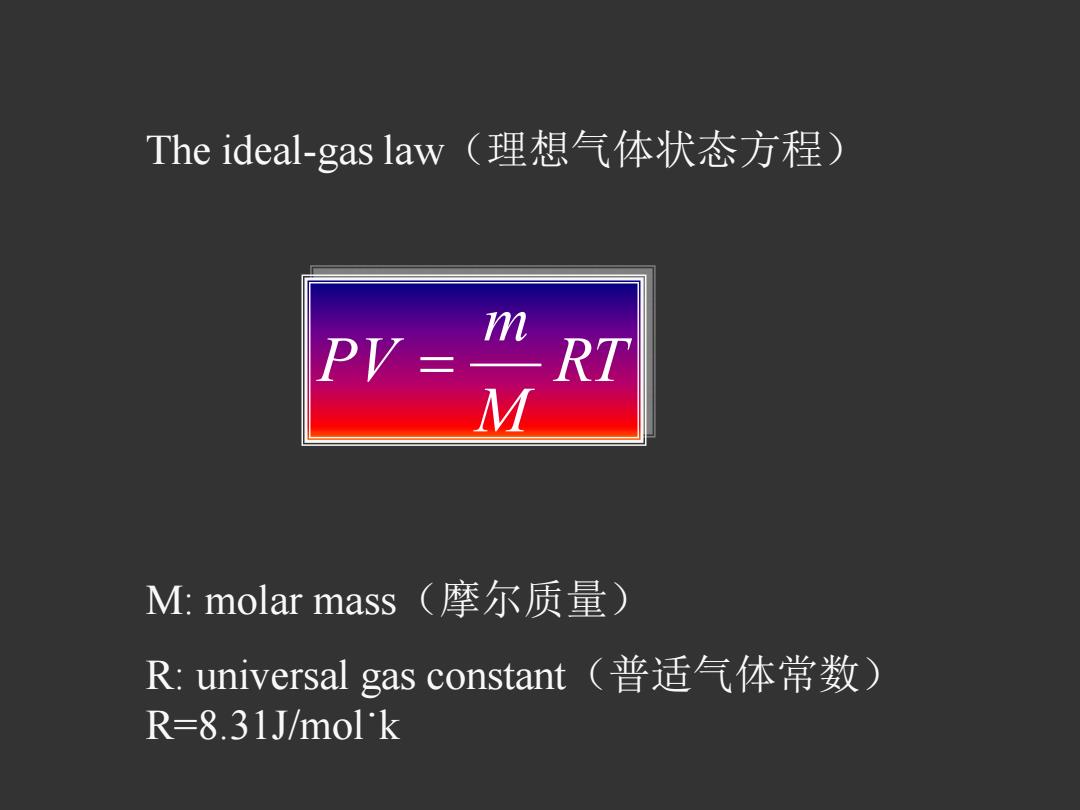
The ideal--gas law(理想气体状态方程) m PV=RT M M:molar mass(摩尔质量) R:universal gas constant(普适气体常数) R=8.31J/mol'k
The ideal-gas law(理想气体状态方程) RT M m PV M: molar mass(摩尔质量) R: universal gas constant(普适气体常数) R=8.31J/molk
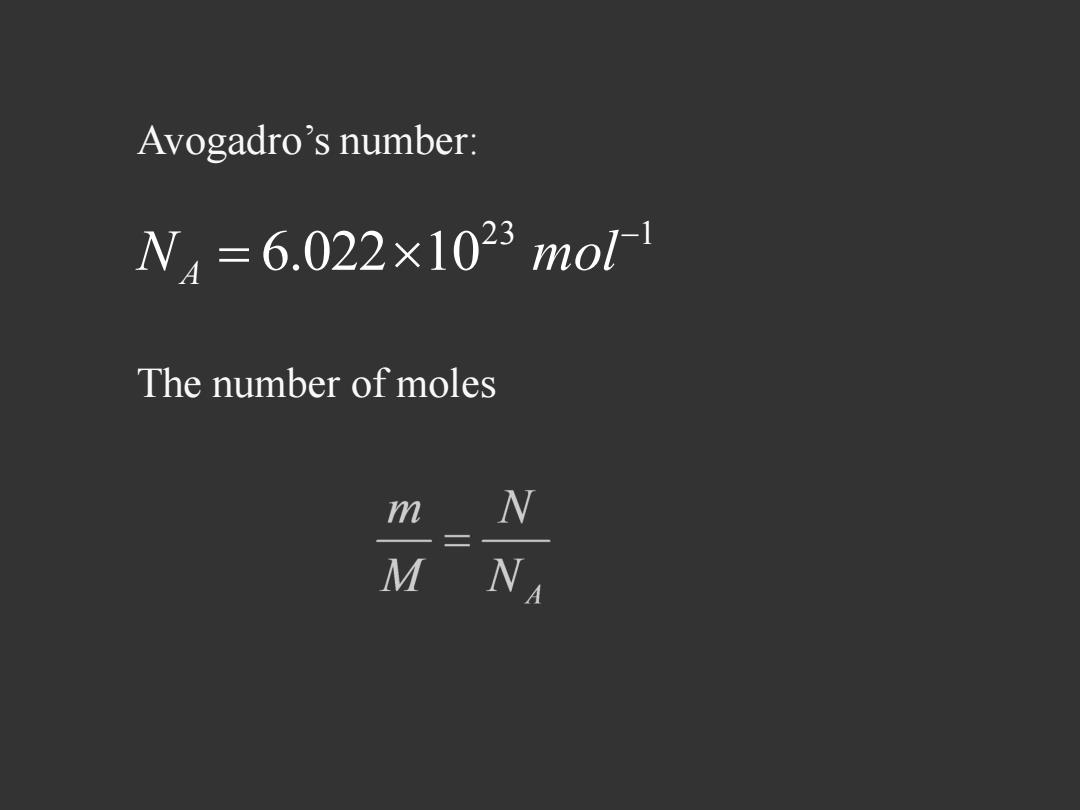
Avogadro's number: N4=6.022×1023mo1 The number of moles N M NA
23 1 6.022 10 N mol A Avogadro’s number: The number of moles
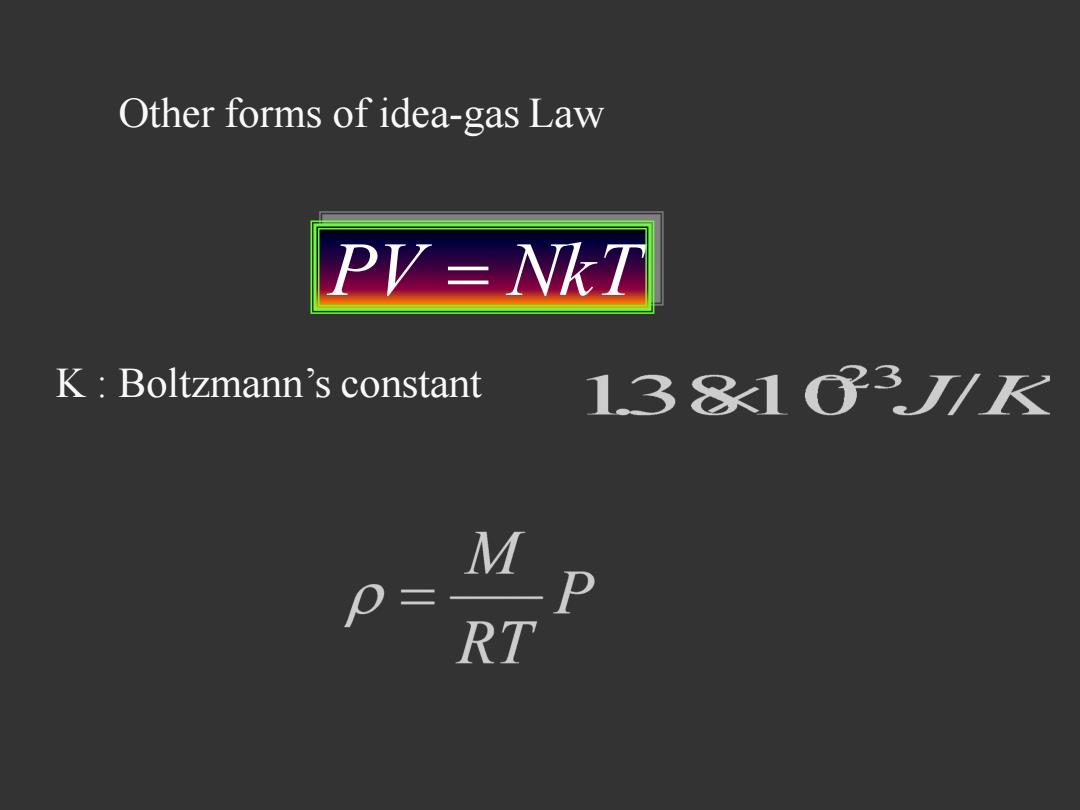
Other forms of idea-gas Law PV-NKT K:Boltzmann's constant 13813/K M 0= RT
PV NkT K : Boltzmann’s constant Other forms of idea-gas Law