
Multi-electron atom Chemical bond
Multi-electron atom Chemical bond

Agenda today 1.Spin of particle 2.the exclusion principle 3.X-ray spectrum 4.The nature of chemical bond
Agenda today 1. Spin of particle 2. the exclusion principle 3. X-ray spectrum 4. The nature of chemical bond

Spin of electron: Force on magnetic dipole in a non-uniform magnetic field ve 4=IS= 元r2 (B 2nr e e mvr= 2m 2m F2=H: @B. 02
Spin of electron: Force on magnetic dipole in a non-uniform magnetic field -e
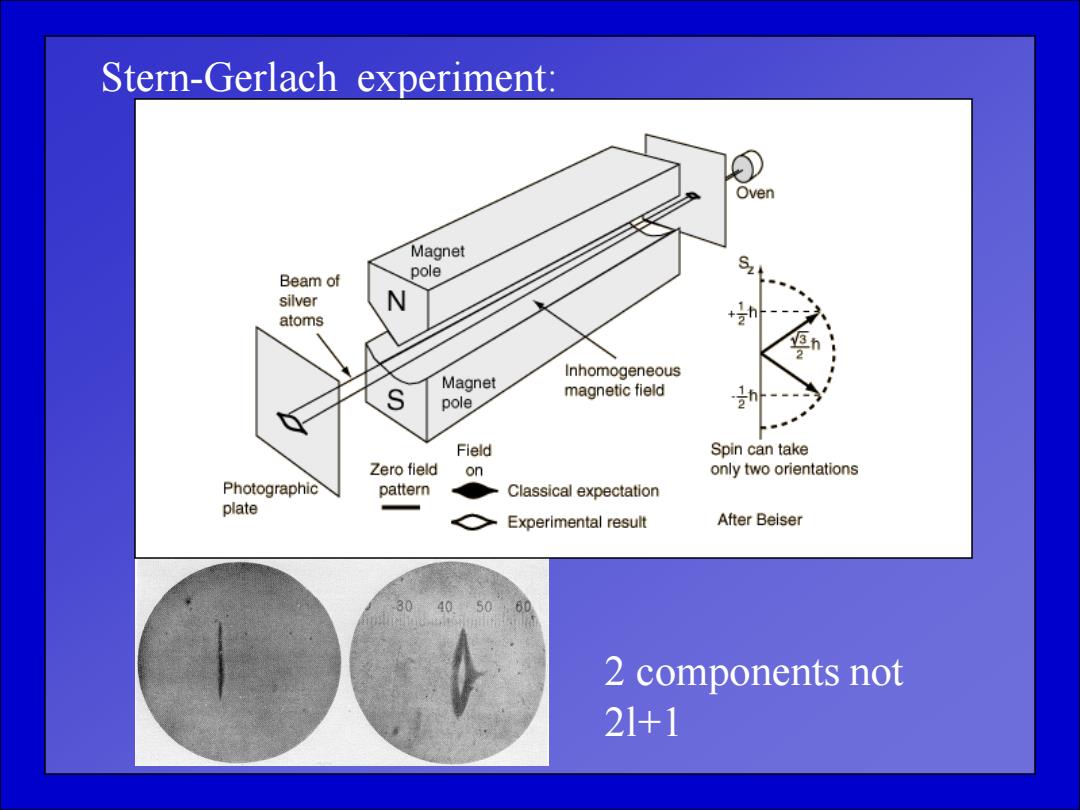
Stern-Gerlach experiment: Oven Magnet Beam of pole silver N atoms Magnet Inhomogeneous S magnetic field pole Field Spin can take Zero field on only two orientations Photographic pattern Classical expectation plate Experimental result After Beiser 2 components not 21+1
Stern-Gerlach experiment: 2 components not 2l+1
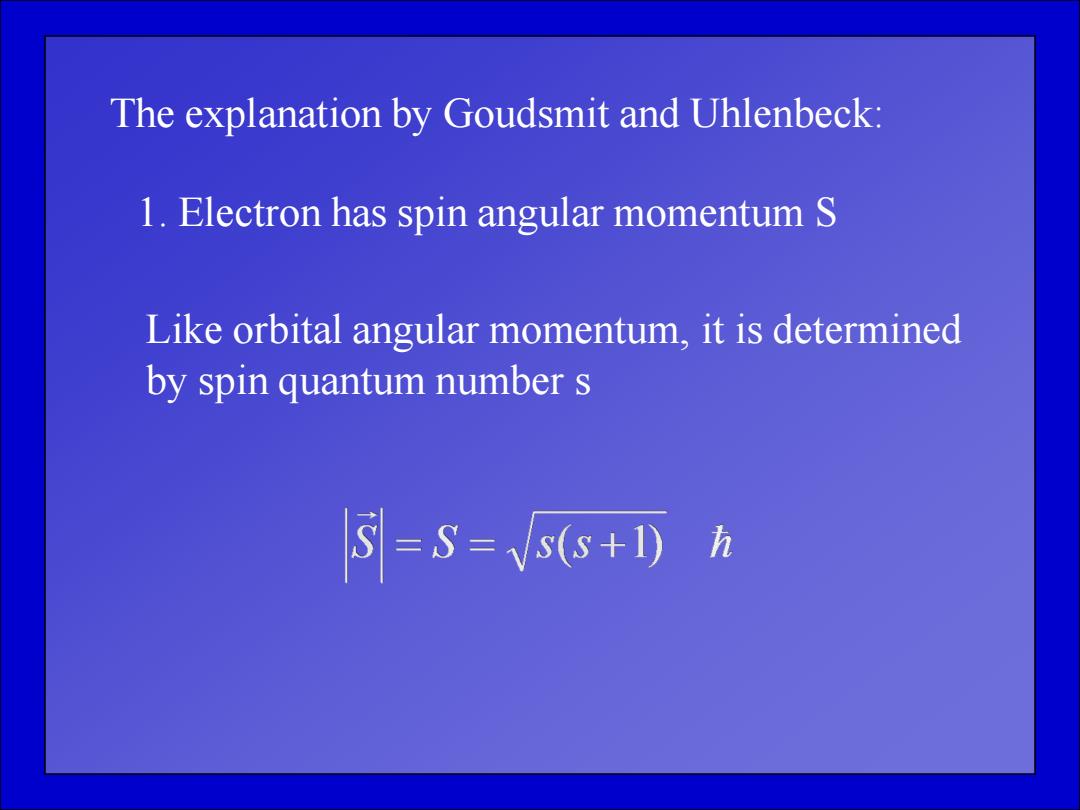
The explanation by Goudsmit and Uhlenbeck: 1.Electron has spin angular momentum S Like orbital angular momentum,it is determined by spin quantum number s =S=5s+D方
The explanation by Goudsmit and Uhlenbeck: 1. Electron has spin angular momentum S Like orbital angular momentum, it is determined by spin quantum number s