The molecular interpretation of temperature: the kinetic theory of gases 1 the gas consists of a large number of molecules that make elastic collisions with each other and with the walls of the container 2 the molecules are separated,on the average, by the distances that are very large compared with their diameters
The molecular interpretation of temperature: the kinetic theory of gases 1 the gas consists of a large number of molecules that make elastic collisions with each other and with the walls of the container 2 the molecules are separated, on the average, by the distances that are very large compared with their diameters

3 the molecules did not exert force on each other except when they collide 4 there is no preferred position for a molecule and no preferred direction for velocity,when there is no external force
3 the molecules did not exert force on each other except when they collide 4 there is no preferred position for a molecule and no preferred direction for velocity, when there is no external force
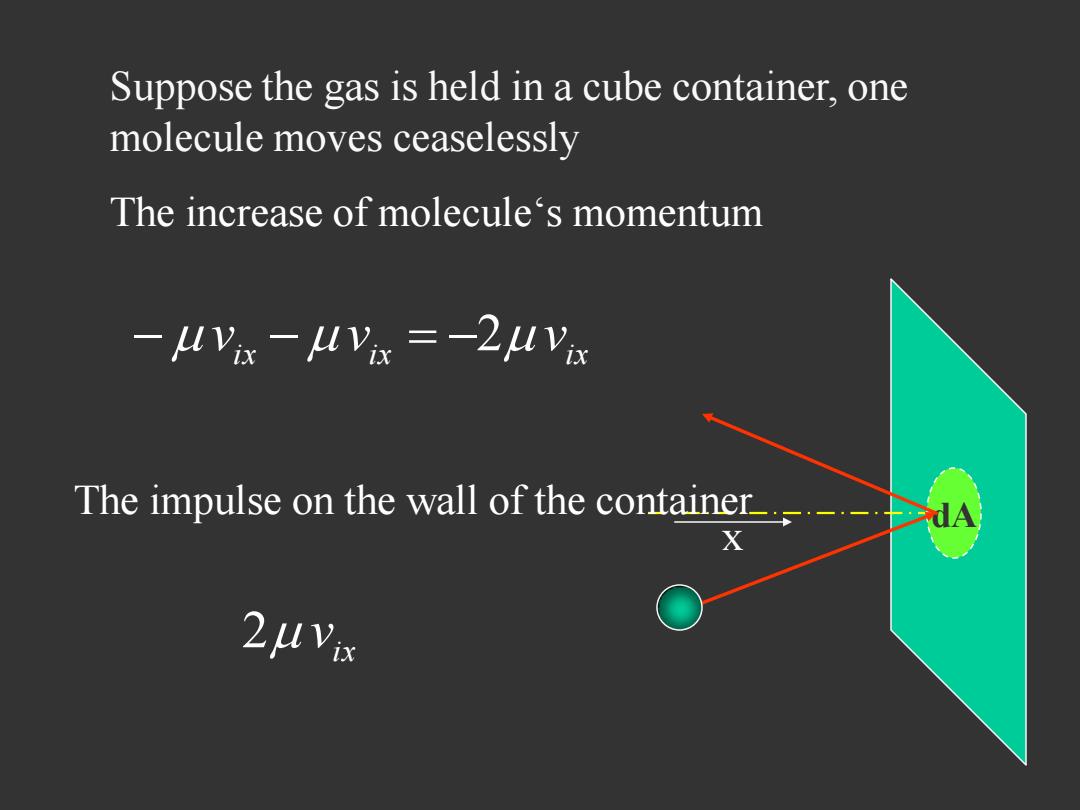
Suppose the gas is held in a cube container,one molecule moves ceaselessly The increase of molecule's momentum -MVis -MlVi =-2MVa The impulse on the wall of the container
x dA The increase of molecule‘s momentum i x i x i x v v 2 v The impulse on the wall of the container ix 2 v Suppose the gas is held in a cube container, one molecule moves ceaselessly
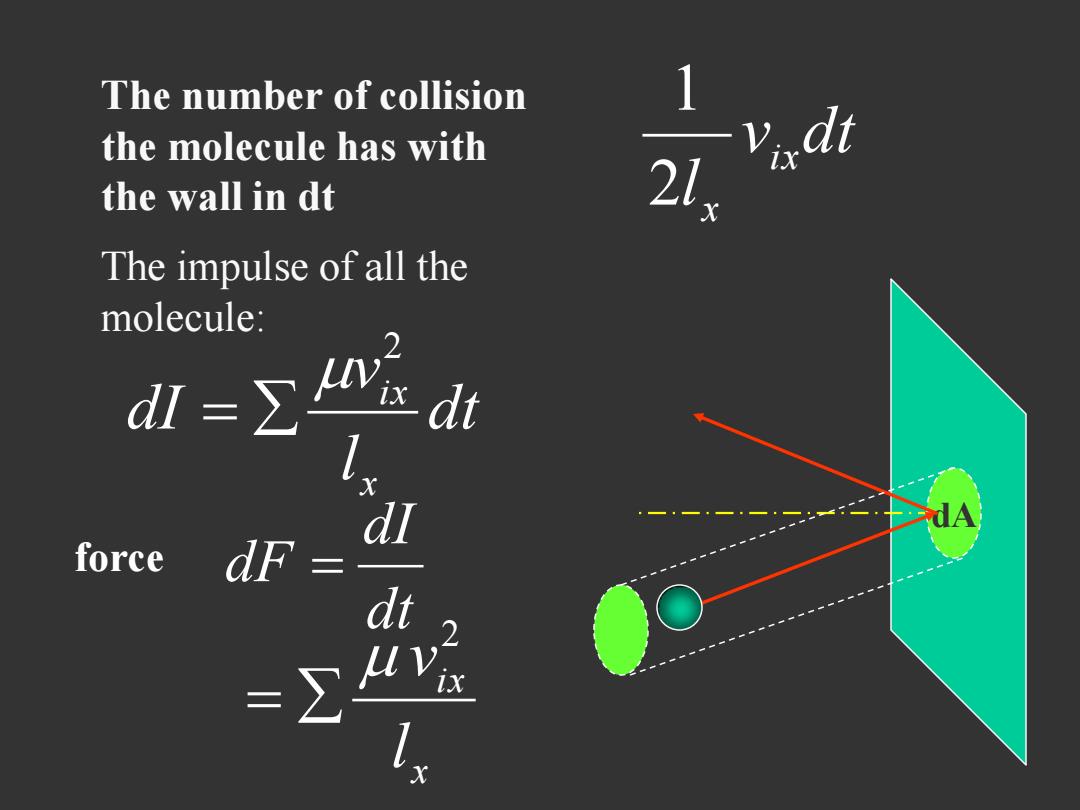
The number of collision the molecule has with the wall in dt The impulse of all the molecule: r dt d force dF dt
dA The number of collision the molecule has with the wall in dt v dt l ix x 2 1 dt l v dI x i x 2 force dt dI dF x ix l v 2 The impulse of all the molecule:
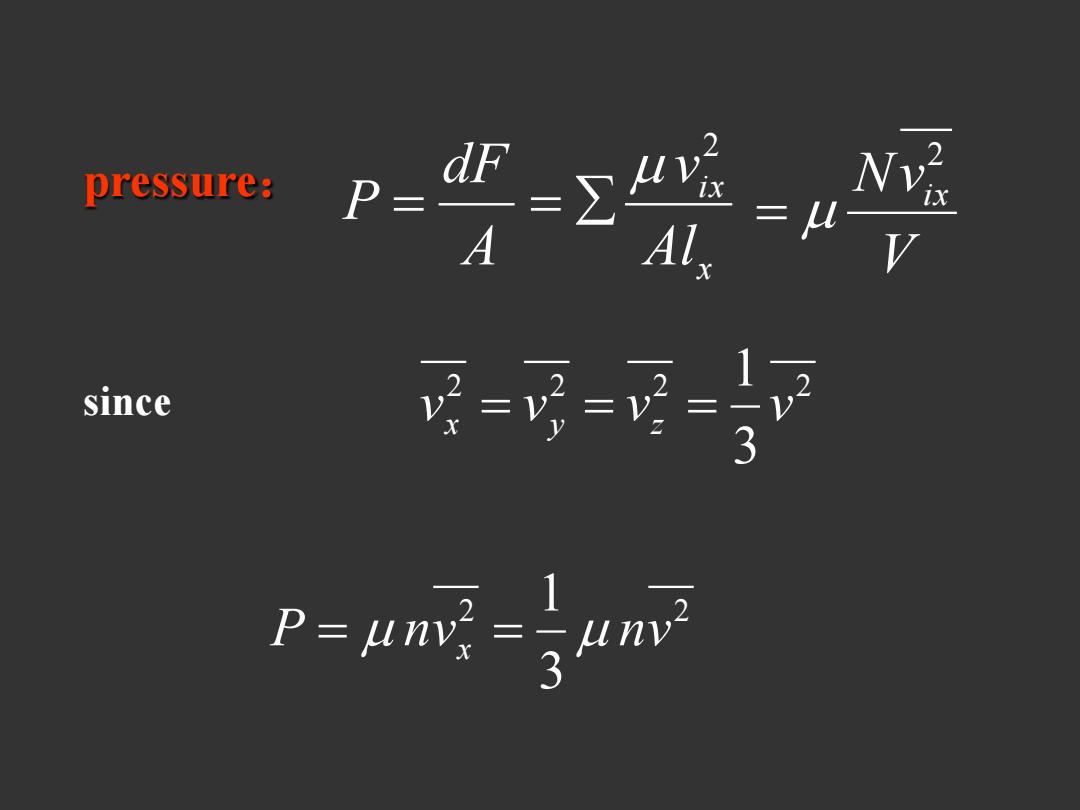
pressure: P=9 since ==- -nn
pressure: x i x Al v A dF P 2 V Nvix 2 since 2 2 2 2 3 1 v v v v x y z 2 2 3 1 P nv nv x