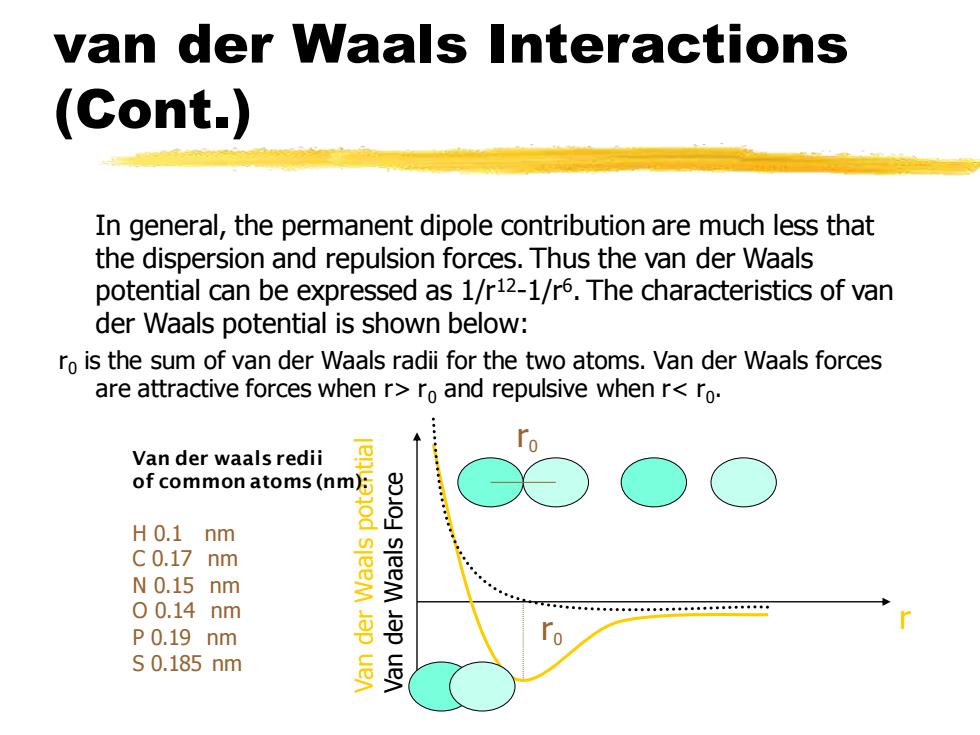
van der Waals Interactions (Cont.) In general,the permanent dipole contribution are much less that the dispersion and repulsion forces.Thus the van der Waals potential can be expressed as 1/r12-1/r6.The characteristics of van der Waals potential is shown below: ro is the sum of van der Waals radii for the two atoms.Van der Waals forces are attractive forces when r>ro and repulsive when r<ro. Van der waals redii of common atoms(nm): H0.1 nm C0.17 nm N0.15 nm sleeM 00.14nm P0.19 nm S0.185nm sleeM Jap uen
van der Waals Interactions (Cont.) In general, the permanent dipole contribution are much less that the dispersion and repulsion forces. Thus the van der Waals potential can be expressed as 1/r12-1/r6 . The characteristics of van der Waals potential is shown below: r0 is the sum of van der Waals radii for the two atoms. Van der Waals forces are attractive forces when r> r0 and repulsive when r< r0 . r Van der Waals potential Van der Waals Force r0 r0 Van der waals redii of common atoms (nm): H 0.1 nm C 0.17 nm N 0.15 nm O 0.14 nm P 0.19 nm S 0.185 nm
3.2 Sequence(primary structure)in Protein Structure All of the information necessary for folding the peptide chain into its "native" structure is contained in the amino acid sequence of the peptide
3.2 • Sequence(primary structure) in Protein Structure All of the information necessary for folding the peptide chain into its “native” structure is contained in the amino acid sequence of the peptide
Primary structure: >The primary structure is the sequence of amino acid residues that constitute the polypeptide chain >Amino acids have the same general structure,but side-chain groups vary in size, shape,charge,hydrophobicity and reactivity
Primary structure: ➢ The primary structure is the sequence of amino acid residues that constitute the polypeptide chain ➢ Amino acids have the same general structure, but side-chain groups vary in size, shape, charge, hydrophobicity and reactivity
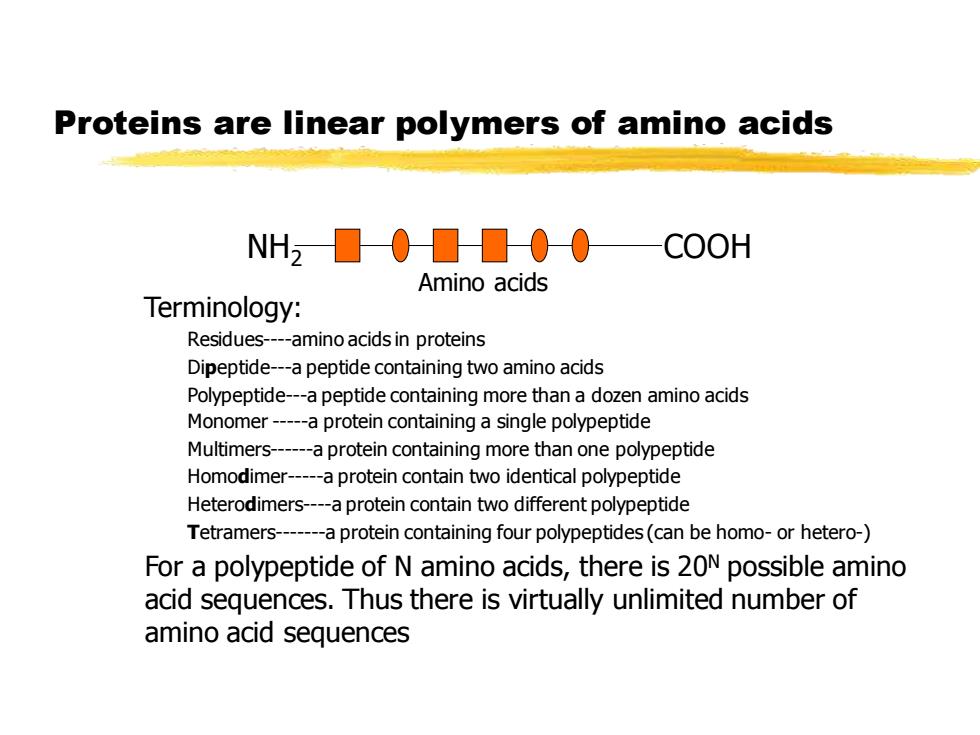
Proteins are linear polymers of amino acids NH2000000 COOH Amino acids Terminology: Residues-amino acids in proteins Dipeptide-a peptide containing two amino acids Polypeptide-a peptide containing more than a dozen amino acids Monomer-a protein containing a single polypeptide Multimers-a protein containing more than one polypeptide Homodimer-a protein contain two identical polypeptide Heterodimers-a protein contain two different polypeptide Tetramers-a protein containing four polypeptides(can be homo-or hetero-) For a polypeptide of N amino acids,there is 20N possible amino acid sequences.Thus there is virtually unlimited number of amino acid sequences
Proteins are linear polymers of amino acids Terminology: Residues-amino acids in proteins Dipeptide-a peptide containing two amino acids Polypeptide-a peptide containing more than a dozen amino acids Monomer -a protein containing a single polypeptide Multimers-a protein containing more than one polypeptide Homodimer-a protein contain two identical polypeptide Heterodimers-a protein contain two different polypeptide Tetramers-a protein containing four polypeptides (can be homo- or hetero-) For a polypeptide of N amino acids, there is 20N possible amino acid sequences. Thus there is virtually unlimited number of amino acid sequences NH2 COOH Amino acids
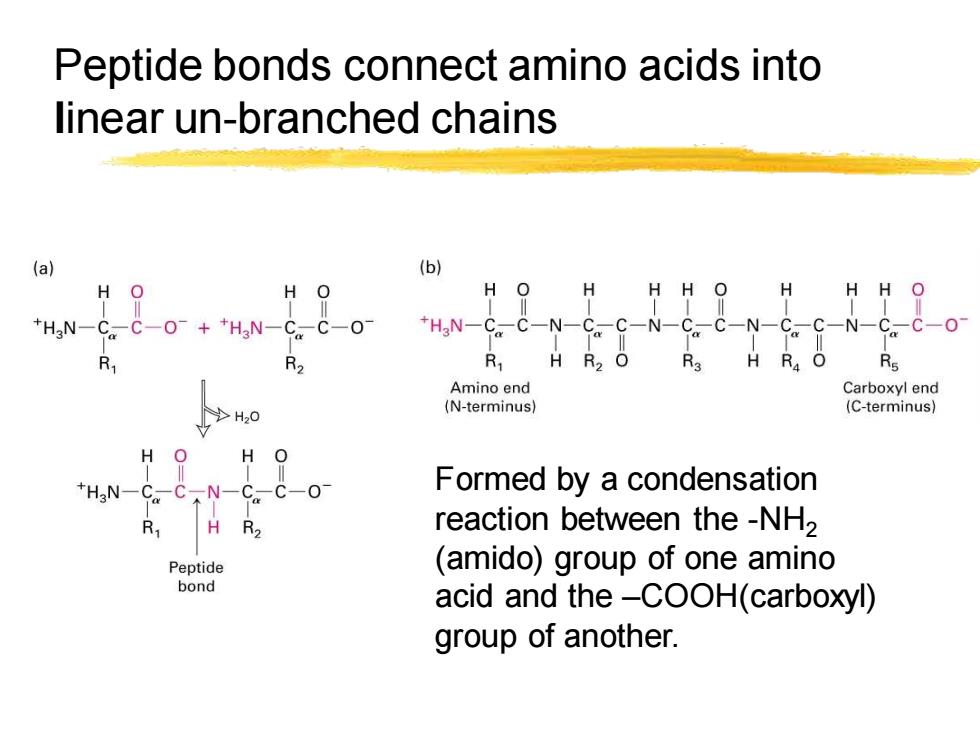
Peptide bonds connect amino acids into linear un-branched chains (a) (b) H H HH O H HH *HgN- -++HN一 HaN-CC-N-C.C-N-C7 ,C-N-C。C-N-C。 R H R O R3 H R.O Rs Amino end Carboxyl end (N-terminus】 (C-terminus) >H20 Formed by a condensation reaction between the -NH2 Peptide (amido)group of one amino bond acid and the-COOH(carboxyl) group of another
Peptide bonds connect amino acids into linear un-branched chains Formed by a condensation reaction between the -NH2 (amido) group of one amino acid and the –COOH(carboxyl) group of another