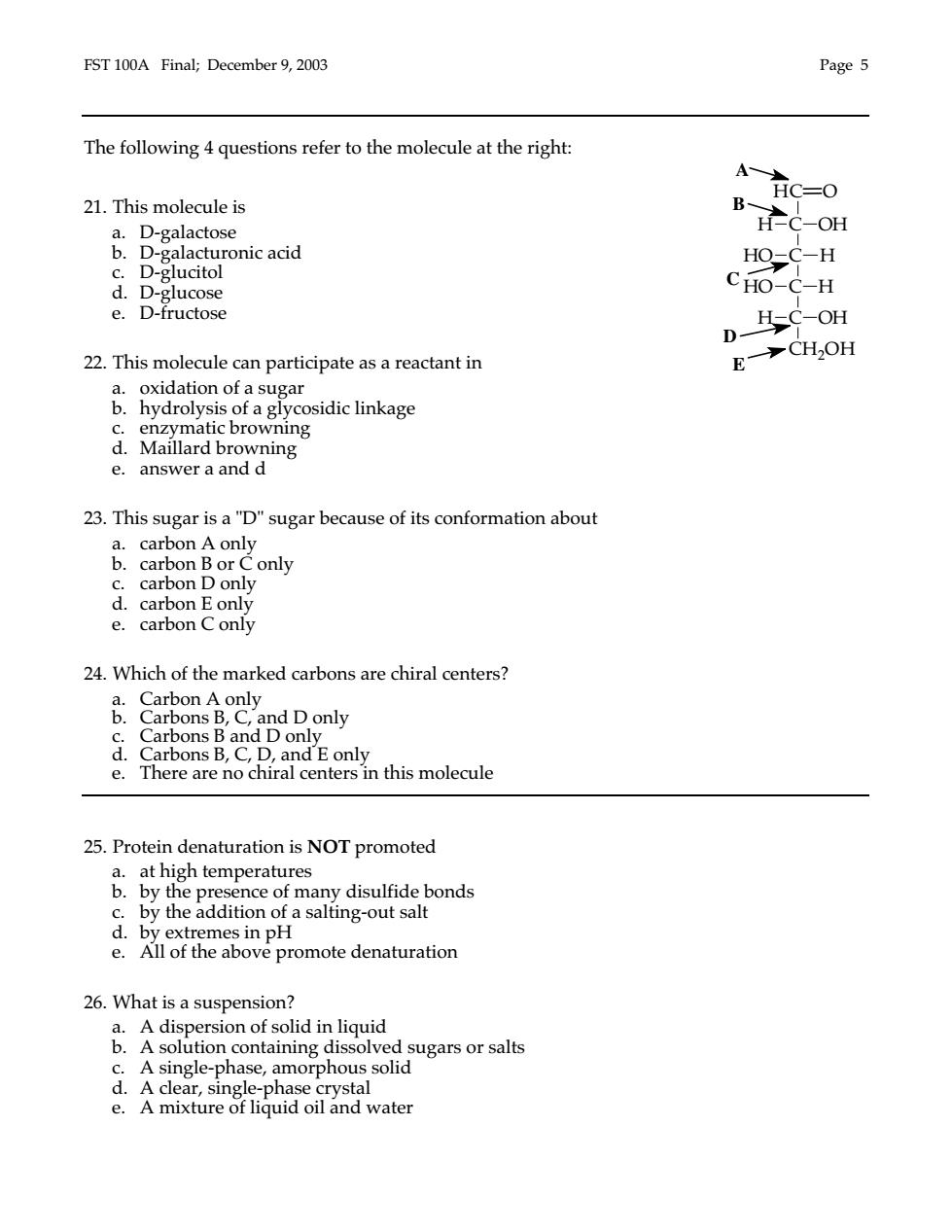
FST 100A Final;December 9,2003 Page 5 The following 4 questions refer to the molecule at the right: 21.This molecule is A-HC-O a.D-galactose HC-OH b.D-galacturonic acid c.D-glucitol HO-C-H d.D-glucose CHO-C-H e.D-fructose 22.This molecule can participate as a reactant in .yryodic inkage a 23.This sugar is a"D"sugar because of its conformation about &Cao B or C only D only e.carbon C only 24.Which of the marked carbons are chiral centers? only e. There are no chiral centers in this molecule 25.Protein denaturation is NOT promoted &时mr ence of many disulfide bonds c.by the addition of a salting-out salt d.by extremes in pH e.All of the above promote denaturation 26.What is a suspension? a.A dispersion of solid in liquid b.A solution containing dissolved sugars or salts c.A single-phase,amorphous solid d.A clear,single -phase rystal e.A mixture of liquid oil and water
FST 100A Final; December 9, 2003 Page 5 The following 4 questions refer to the molecule at the right: 21. This molecule is a. D-galactose b. D-galacturonic acid c. D-glucitol d. D-glucose e. D-fructose 22. This molecule can participate as a reactant in a. oxidation of a sugar b. hydrolysis of a glycosidic linkage c. enzymatic browning d. Maillard browning e. answer a and d 23. This sugar is a "D" sugar because of its conformation about a. carbon A only b. carbon B or C only c. carbon D only d. carbon E only e. carbon C only 24. Which of the marked carbons are chiral centers? a. Carbon A only b. Carbons B, C, and D only c. Carbons B and D only d. Carbons B, C, D, and E only e. There are no chiral centers in this molecule 25. Protein denaturation is NOT promoted a. at high temperatures b. by the presence of many disulfide bonds c. by the addition of a salting-out salt d. by extremes in pH e. All of the above promote denaturation 26. What is a suspension? a. A dispersion of solid in liquid b. A solution containing dissolved sugars or salts c. A single-phase, amorphous solid d. A clear, single-phase crystal e. A mixture of liquid oil and water E D C B A C C C C HC OH H OH HO H H HO H CH2OH O

FST 100A Final;December 9,2003 Page 6 .ho blw Of the mnoderno A B CH:-CH-CH2- 〉-CH2 OH-CH,- CH .Reidue con Residues B and C only d.Residues A and B o e.ResiduesA Band 28.Why do food scientists modify cellulose by attaching carboxymethyl groups? c.To increase r oulsion between solutes d.Answer b and c e.All of the above are correct reasons 29.Which of the following are common features of amylose and cellulose? a.They both are glucans (chains of glucose subunits) y both are easily dig sted c.They both form helical structures d.They are both highly water soluble e.All of the above are common features 30.Starch in potatoes,corn or wheat a.is dissolved in aqueous solution b.is found in solid,semicrystalline granules c.contains only amylose d.contains a mixture of amylopectin and cellulose e.forms long,strong linear fibers which give structure to the plant 31.Which of the following(a-d)is NOT true of an enediol?(Choose"e"if all are true) a.It can be created during isomerization reactions b. It is produced b y adding a proton to the enolate ion It is an intermec iate in the isomerization of glucose to fructose
FST 100A Final; December 9, 2003 Page 6 27. Three amino acid R groups are shown below. Of the amino acids bearing these groups, which is hydrophobic? a. Residue B only b. Residue C only c. Residues B and C only d. Residues A and B only e. Residues A, B and C 28. Why do food scientists modify cellulose by attaching carboxymethyl groups? a. To make it sweeter b. To enhance its water solubility c. To increase repulsion between solutes d. Answer b and c e. All of the above are correct reasons 29. Which of the following are common features of amylose and cellulose? a. They both are glucans (chains of glucose subunits) b. They both are easily digested c. They both form helical structures d. They are both highly water soluble e. All of the above are common features 30. Starch in potatoes, corn or wheat a. is dissolved in aqueous solution b. is found in solid, semicrystalline granules c. contains only amylose d. contains a mixture of amylopectin and cellulose e. forms long, strong linear fibers which give structure to the plant 31. Which of the following (a-d) is NOT true of an enediol? (Choose "e" if all are true) a. It can be created during isomerization reactions b. It is produced by adding a proton to the enolate ion c. It is an intermediate in the isomerization of glucose to fructose d. It contains a carbon-carbon double bond e. All of the above are true of an enediol C OH CH2 B CH2 A CH3 CH CH2 CH3