
Name:KEY Last First Signature: INSTRUCTIONS PRINT your last and first names above.Then SIGN the exam copy,to indicate that You have respected the UC Davis Honor Code in the taking of this exam. PRINT your name on the front of your Scantron #2000,and mark the test version that appears at the top of this page on your Scantron2000. wREy2stnntemtoiomaHeheraohaeRBC8OEvaSOibrmit bubbles. CHECK that there are 36 multiple choice questions.There are a total of 11 pages to the exam. Use a #2 pencil to mark your Scantron answers.You are responsible for the accuracy and completeness of your Scantron.Do not put any marks (other than filling in the answer bubbles)on the left side(s)of the Scantron sheet. Graded exams will be returned in class as soon as possible. Multiple Choice 90 t answers 100
Food Science & Technology 100A Professor S.R. Dungan Midterm Examination #1 October 22, 2003 Name:KEY Last First Signature: INSTRUCTIONS PRINT your last and first names above. Then SIGN the exam copy, to indicate that You have respected the UC Davis Honor Code in the taking of this exam. PRINT your name on the front of your Scantron #2000, and mark the test version that appears at the top of this page on your Scantron #2000. WRITE your student identification number in the appropriate area of your Scantron #2000 on Part 1 and follow the instructions to CODE your ID number in the bubbles. CHECK that there are 36 multiple choice questions. There are a total of 11 pages to the exam. Use a #2 pencil to mark your Scantron answers. You are responsible for the accuracy and completeness of your Scantron. Do not put any marks (other than filling in the answer bubbles) on the left side(s) of the Scantron sheet. Graded exams will be returned in class as soon as possible. Multiple Choice 90 Short answers 10 Total 100

FST100A2003 Midterm1 1.Which of the following correctly describes a triglyceride molecule? a.It consists of three acyl chains connected to a glycerol backbone@ b.It has three chiral centers c.It has three fatty acid chains attached to a glycose subunit d.It has two fatty acid groups and an isoprene group e.All of the above correctly describe a triglyceride molecule 2.Which of the following are true of sugar alcohols? a.They are often called"uronic acids" b.They are good humectants c.They are produced by reduction of sugars d.They have0 calories e.Answer b and c@ 3.Which of the following pairs are diastereomers? a.B-D-glucopyranose and B-D-glucofuranose b.The straight chain forms of D-glucose and D-fructose c.a-D-glucopyranose and B-D-galactopyranose@ d.The straight chain form of D-glucose and D-glucitol e.a-D-glucopyranose and B-D-lactose 4.Why are monosaccharides and disaccharides added to foods? a.To increase the food's sweetness@ b.To increase the food's bitterness c.Because they are very hydrophobic d.Because they prevent enzymatic browning e.All of the above are reasons 5.Which of the following is a lipid? a.limonene b.phosphatidylcholine c.molasses d.triacylglycerols e.answers a,b and d@
FST 100A 2003 Midterm 1 1 1. Which of the following correctly describes a triglyceride molecule? a. It consists of three acyl chains connected to a glycerol backbone @ b. It has three chiral centers c. It has three fatty acid chains attached to a glycose subunit d. It has two fatty acid groups and an isoprene group e. All of the above correctly describe a triglyceride molecule 2. Which of the following are true of sugar alcohols? a. They are often called "uronic acids" b. They are good humectants c. They are produced by reduction of sugars d. They have 0 calories e. Answer b and c @ 3. Which of the following pairs are diastereomers? a. β-D-glucopyranose and β-D-glucofuranose b. The straight chain forms of D-glucose and D-fructose c. α-D-glucopyranose and β-D-galactopyranose @ d. The straight chain form of D-glucose and D-glucitol e. α-D-glucopyranose and β-D-lactose 4. Why are monosaccharides and disaccharides added to foods? a. To increase the food's sweetness @ b. To increase the food's bitterness c. Because they are very hydrophobic d. Because they prevent enzymatic browning e. All of the above are reasons 5. Which of the following is a lipid? a. limonene b. phosphatidylcholine c. molasses d. triacylglycerols e. answers a, b and d @
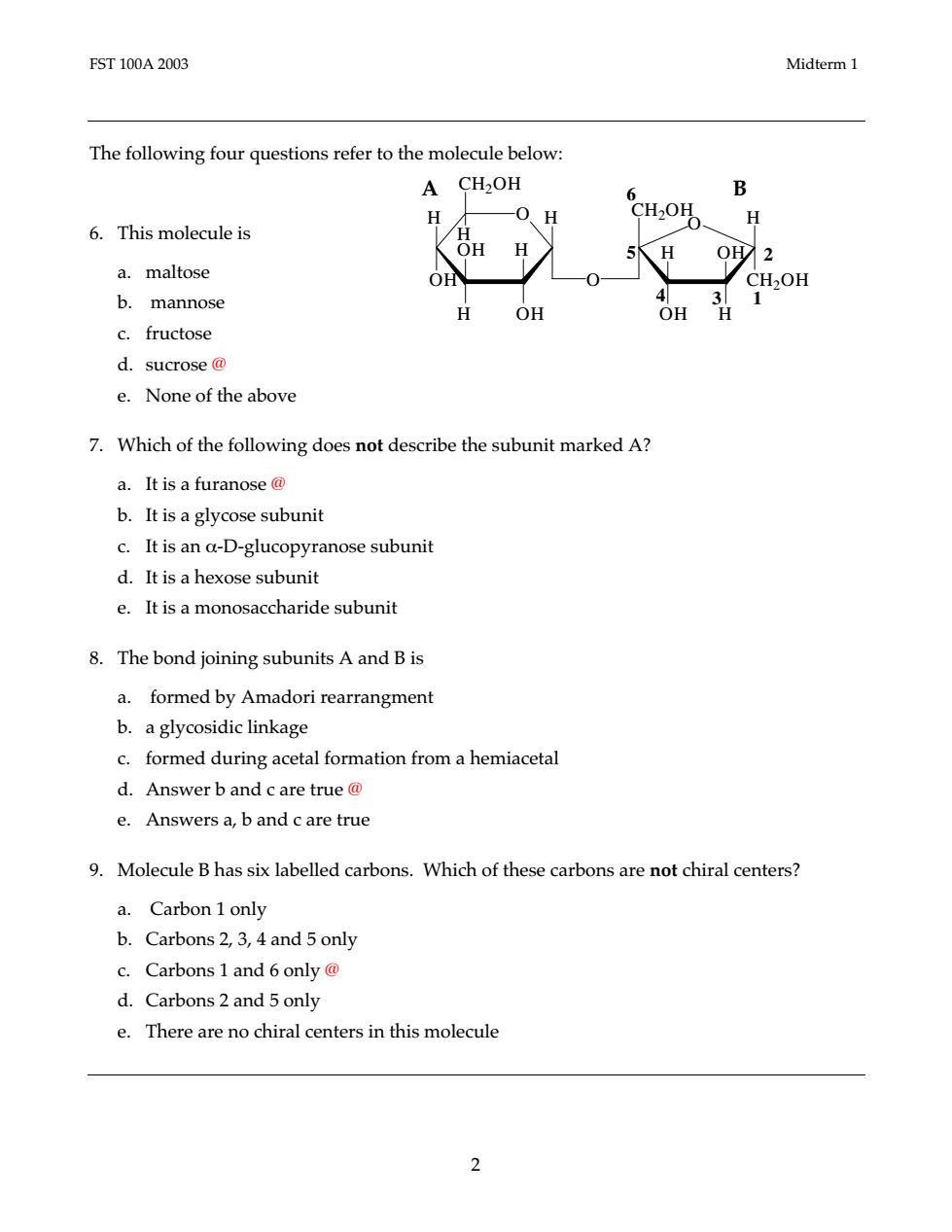
FST100A2003 Midterm1 The following four questions refer to the molecule below: A CH2OH B 6.This molecule is 0、H CH2OH H 、OHH a.maltose OH b.mannose H OH OH c.fructose d.sucrose@ e.None of the above 7.Which of the following does not describe the subunit marked A? a.It is a furanose@ b.It is a glycose subunit c.It is an a-D-glucopyranose subunit d.It is a hexose subunit e.It is a monosaccharide subunit 8.The bond joining subunits A and B is a.formed by Amadori rearrangment b.a glycosidic linkage c.formed during acetal formation from a hemiacetal d.Answer b and c are true@ e.Answers a,b and c are true 9.Molecule B has six labelled carbons.Which of these carbons are not chiral centers? a.Carbon 1 only b.Carbons 2,3,4 and 5 only c.Carbons 1 and 6 only@ d.Carbons 2 and 5 only e.There are no chiral centers in this molecule 2
FST 100A 2003 Midterm 1 2 The following four questions refer to the molecule below: 6. This molecule is a. maltose b. mannose c. fructose d. sucrose @ e. None of the above 7. Which of the following does not describe the subunit marked A? a. It is a furanose @ b. It is a glycose subunit c. It is an α-D-glucopyranose subunit d. It is a hexose subunit e. It is a monosaccharide subunit 8. The bond joining subunits A and B is a. formed by Amadori rearrangment b. a glycosidic linkage c. formed during acetal formation from a hemiacetal d. Answer b and c are true @ e. Answers a, b and c are true 9. Molecule B has six labelled carbons. Which of these carbons are not chiral centers? a. Carbon 1 only b. Carbons 2, 3, 4 and 5 only c. Carbons 1 and 6 only @ d. Carbons 2 and 5 only e. There are no chiral centers in this molecule A B O O OH H OH H H O H H H OH H OH H OH CH2OH CH2OH CH2OH 4 3 1 5 6 2

FST100A2003 Midterm1 10.Isomerization reactions are used a.to remove fructose from corn syrup b.to convert D-glucose to D-galactose c.to convert sucrose to invert sugar d.by bees to increase the fructose content of honey@ e.Answer a and d 11.Most abundant,natural sugars are a.ketoses b.hexoses@ c.furanoses d.L-sugars e.Most sugars are all of the above 12.Which of the following explains the very high purity of table sugar? a.Table sugar is primarily fructose,a pure sugar found in fruit b.Table sugar is made from sucrose,which crystallizes very easily@ c.Table sugar is made from sucrose,which is very hygroscopic d.Table sugar is made from L-sugars,which are naturally of high purity e.All of the above 13.Glucose is sometimes called a.grape sugar b.dextrose c.corn sugar d.Answer b and c e.Answer a,b and c@ 14.Hydrolysis reactions are used to a.form disaccharides from monosaccharides b.create invert sugar from sucrose@ c.convert glucose to mannose d.oxidize phenolics e.produce sugar alcohols
FST 100A 2003 Midterm 1 3 10. Isomerization reactions are used a. to remove fructose from corn syrup b. to convert D-glucose to D-galactose c. to convert sucrose to invert sugar d. by bees to increase the fructose content of honey @ e. Answer a and d 11. Most abundant, natural sugars are a. ketoses b. hexoses @ c. furanoses d. L-sugars e. Most sugars are all of the above 12. Which of the following explains the very high purity of table sugar? a. Table sugar is primarily fructose, a pure sugar found in fruit b. Table sugar is made from sucrose, which crystallizes very easily @ c. Table sugar is made from sucrose, which is very hygroscopic d. Table sugar is made from L-sugars, which are naturally of high purity e. All of the above 13. Glucose is sometimes called a. grape sugar b. dextrose c. corn sugar d. Answer b and c e. Answer a, b and c @ 14. Hydrolysis reactions are used to a. form disaccharides from monosaccharides b. create invert sugar from sucrose @ c. convert glucose to mannose d. oxidize phenolics e. produce sugar alcohols

FST100A2003 Midterm1 15.Hexanoic acid is a fatty acid with a.6 carbons@ b.12 carbons c.one double bond d.two double bonds e.none of the above 16.Fructose is the only sugar found in a.sugar beets b.apples c.peaches d.tomatos e.All of the above would contain sugars besides fructose 17.Which of the following fatty acid groups are seldom found in food triglycerides? a.oleic acid b.18 carbon fatty acids c.monounsaturated fatty acids with cis double bonds d.answer a and c e.answers a,b and c are all found commonly in foods@ 18.Why are apples stored under modified atmosphere? a.to prevent conversion of sugar to sugar alcohols b.to prevent loss of essential amino acids due to Maillard browning c.to prevent oxygen from promoting enzymatic browning@ d.to prevent loss of erucic acid e.answers b and c 19.Which of the following is involved in caramelization? a.formation of quinones b.acetal formation c.Amadori rearrangement(isomerization)of an imine d.nucleophilic addition of an amine e.none of the above@ 4
FST 100A 2003 Midterm 1 4 15. Hexanoic acid is a fatty acid with a. 6 carbons @ b. 12 carbons c. one double bond d. two double bonds e. none of the above 16. Fructose is the only sugar found in a. sugar beets b. apples c. peaches d. tomatos e. All of the above would contain sugars besides fructose @ 17. Which of the following fatty acid groups are seldom found in food triglycerides? a. oleic acid b. 18 carbon fatty acids c. monounsaturated fatty acids with cis double bonds d. answer a and c e. answers a, b and c are all found commonly in foods @ 18. Why are apples stored under modified atmosphere? a. to prevent conversion of sugar to sugar alcohols b. to prevent loss of essential amino acids due to Maillard browning c. to prevent oxygen from promoting enzymatic browning @ d. to prevent loss of erucic acid e. answers b and c 19. Which of the following is involved in caramelization? a. formation of quinones b. acetal formation c. Amadori rearrangement (isomerization) of an imine d. nucleophilic addition of an amine e. none of the above @