
Food Science Technology 100A Professor S.R.Dungan First Midterm Examination October 27,2004 Version A Name:KEY Last First Signature: INSTRUCTIONS PRINT your last and first names above.Then SIGN the exam copy. PRINT your name on the front of your Scantron #2000. WRITE the test version that appears at the top of this page onyour Scantron000. WRITE your student identification number in the appropriate area of your Scantron #2000 on Part 1 and follow the instructions to CODE your ID number in the bubbles. CHECK that there are 36 multiple choice questions.There are a total of 8 pages to the exam. complete t your Scantron Please respect the UC Davis Honor Code in the taking of this exam. There should be plenty of time to take this exam without being rushed.However,you will need to use your time efficiently. Graded exams can be picked up in 110 FSTB. Multiple Choice /g0 /1 answers Total 100
Food Science & Technology 100A Professor S.R. Dungan First Midterm Examination October 27, 2004 Version A Name:KEY Last First Signature: INSTRUCTIONS PRINT your last and first names above. Then SIGN the exam copy. PRINT your name on the front of your Scantron #2000. WRITE the test version that appears at the top of this page on your Scantron #2000. WRITE your student identification number in the appropriate area of your Scantron #2000 on Part 1 and follow the instructions to CODE your ID number in the bubbles. CHECK that there are 36 multiple choice questions. There are a total of 8 pages to the exam. Use a #2 pencil to mark your Scantron answers. You are responsible for the accuracy and completeness of your Scantron. Do not put any marks (other than filling in the answer bubbles) on the left side(s) of the Scantron sheet. Please respect the UC Davis Honor Code in the taking of this exam. There should be plenty of time to take this exam without being rushed. However, you will need to use your time efficiently. Graded exams can be picked up in 110 FSTB. Multiple Choice /90 Short answers / 10 Total 100
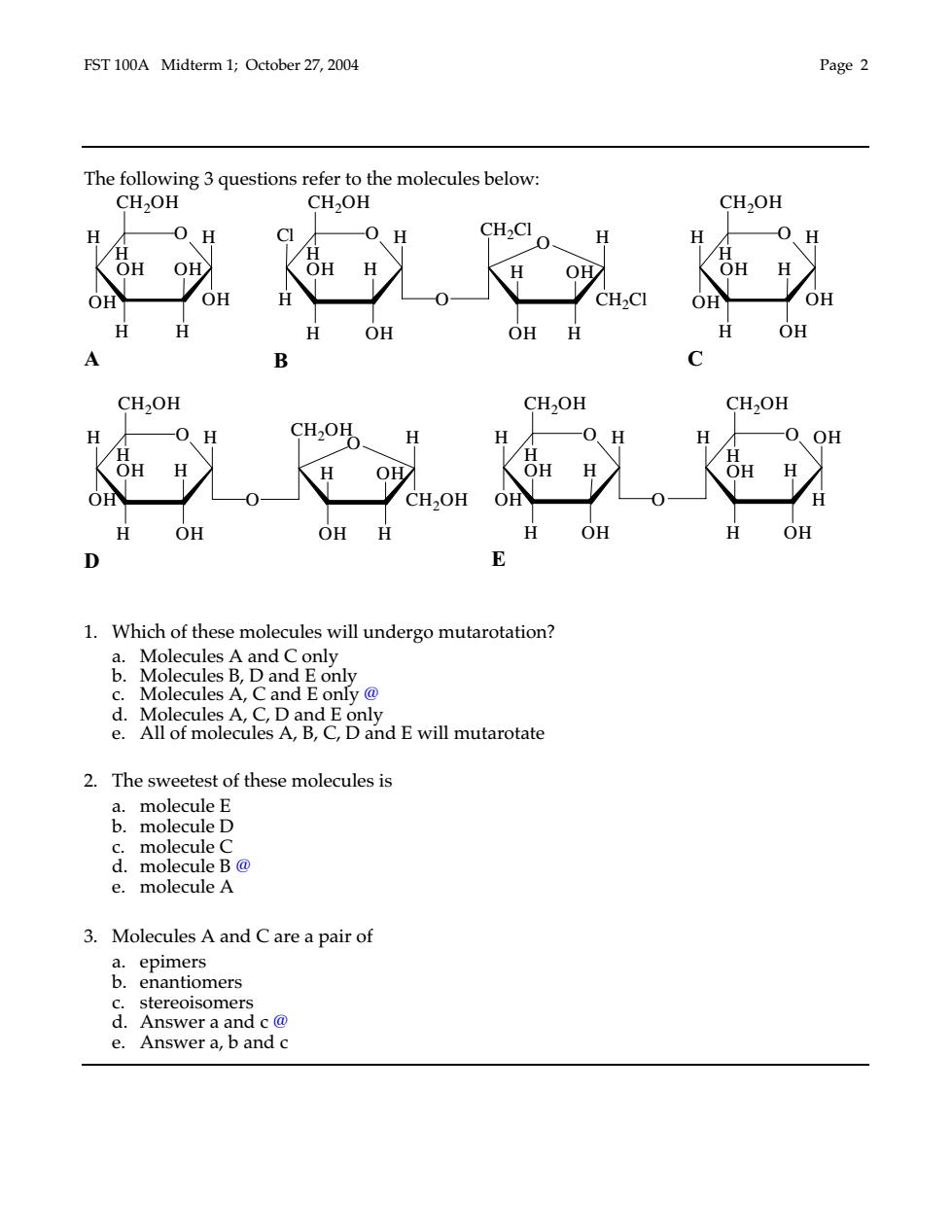
FST 100A Midterm 1;October 27,2004 Page2 The following 3 questions refer to the molecules below: CH2OH CH2OH H CH2CI O OH OH OH H OH OH H B CH2OH CH2OH CH2OH H CH2OHO O OH OH H OH H O 0 CH2OH OH H OH OH H H OH OH D 1.Which of these molecules will undergo mutarotation? Molecules A and C only Molecules B,D and on .D and E will mutarotate 2.The sweetest of these molecules is B.molcule D c.molecule C d.molecule B@ e.molecule A 3.Molecules A and C are a pair of a.epimers b.enantiomers stereoisomers Answer a and c@ e.Answer a,b and c
FST 100A Midterm 1; October 27, 2004 Page 2 The following 3 questions refer to the molecules below: O OH H H H OH H OH CH2OH H OH A O O OH H OH H Cl O H H H OH H OH H H CH2Cl CH2OH CH2Cl B O OH H H H H OH OH CH2OH H OH C O O OH H OH H H O H H H OH H OH H OH CH2OH CH2OH CH2OH D O O H H H H H H O H OH H H OH OH OH OH OH CH2OH CH2OH H E 1. Which of these molecules will undergo mutarotation? a. Molecules A and C only b. Molecules B, D and E only c. Molecules A, C and E only @ d. Molecules A, C, D and E only e. All of molecules A, B, C, D and E will mutarotate 2. The sweetest of these molecules is a. molecule E b. molecule D c. molecule C d. molecule B @ e. molecule A 3. Molecules A and C are a pair of a. epimers b. enantiomers c. stereoisomers d. Answer a and c @ e. Answer a, b and c
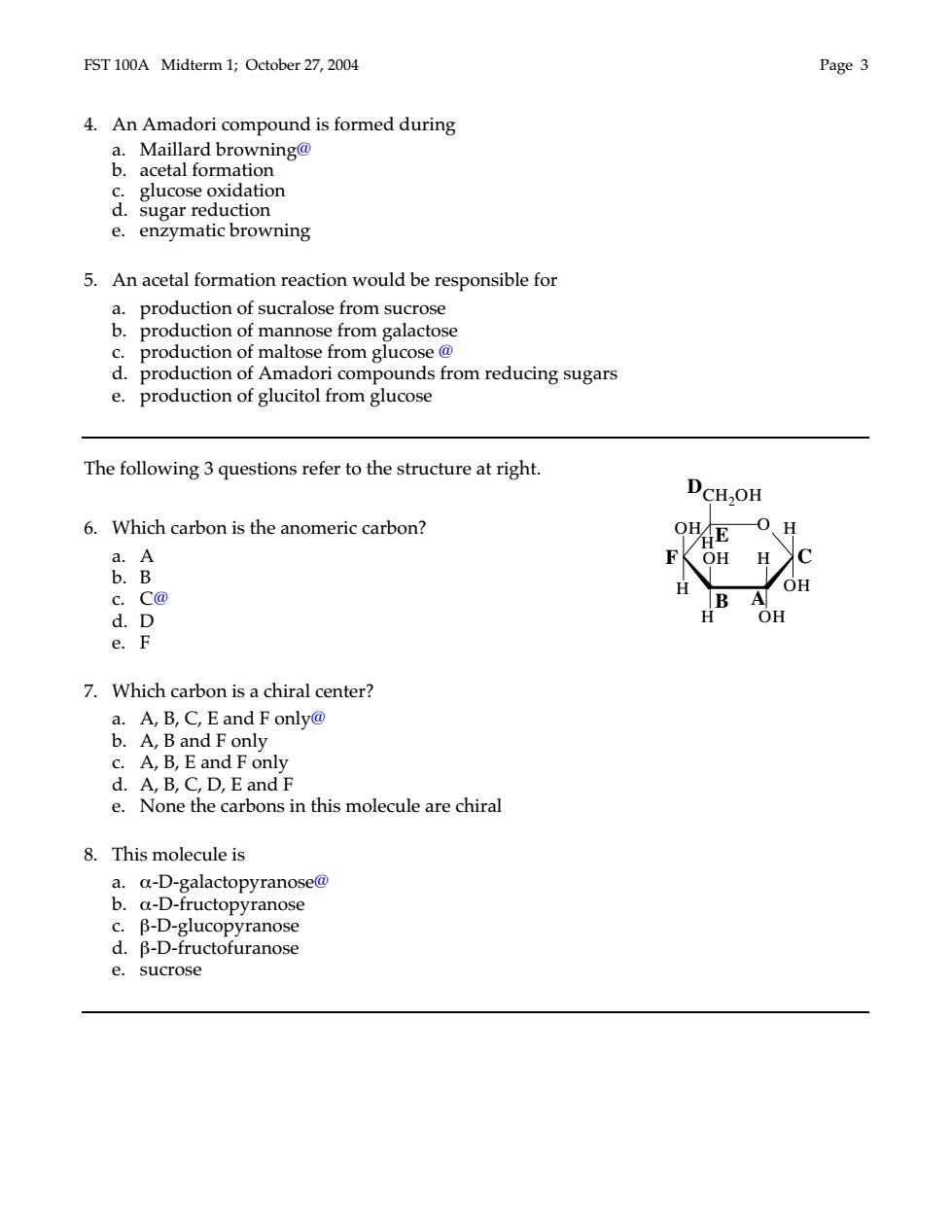
FST 100A Midterm 1;October 27,2004 Page 3 4.An Amadori compound is formed during &.adatadhaomng9 d.reducor glucose oxidation e.enzymatic browning 5.An acetal formation reaction would be responsible for production of sucralose from sucrose dcti。 pro n of a e from galacto pro n c rom glucos on o mado 1 compound is from reducing sugars e.production of glucitol from glucose The following 3 questions refer to the structure at right. DCH-OH 6.Which carbon is the anomeric carbon? OHE 0、H a.A OH H b.B H OH c.C@ d.D H OH e.F 7.Which carbon is a chiral center? A,B,C,Eand Fonly@ c.A,B,E and F only d.A,B,C,D,E and F e.None the carbons in this molecule are chiral 8.This molecule is a.a-D-galactopyranose@ b. -D-fructopyran e sucrose
FST 100A Midterm 1; October 27, 2004 Page 3 4. An Amadori compound is formed during a. Maillard browning@ b. acetal formation c. glucose oxidation d. sugar reduction e. enzymatic browning 5. An acetal formation reaction would be responsible for a. production of sucralose from sucrose b. production of mannose from galactose c. production of maltose from glucose @ d. production of Amadori compounds from reducing sugars e. production of glucitol from glucose The following 3 questions refer to the structure at right. 6. Which carbon is the anomeric carbon? a. A b. B c. C@ d. D e. F 7. Which carbon is a chiral center? a. A, B, C, E and F only@ b. A, B and F only c. A, B, E and F only d. A, B, C, D, E and F e. None the carbons in this molecule are chiral 8. This molecule is a. α-D-galactopyranose@ b. α-D-fructopyranose c. β-D-glucopyranose d. β-D-fructofuranose e. sucrose O OH H H OH H OH H CH2OH H OH B A C D E F

FST 100A Midterm 1;October 27,2004 Page 4 9.Which of the following would NOT contribute calories? c.Maltodextrins d.Sucrose e.All of the above would contribute calories@ 10.A particular sugar alcohol has a relative sweetness intensity of f=0.5.We would therefore expect that a.10 g of the sugar alcohol would be as sweet as 10 g of sucrose in a food b.10 g of the sugar alcohol would be as sweet as 20 g of sucrose in a food c.10 g of the sugar alcohol would be as sweet as 0.5 g of sucrose in a food d.20 g of the sugar alcohol would be as sweet as 10g of sucrose in a food e.the sugar alcohol does not have a sweet taste 11.Which of the following is sweeter than sucrose at room temperature? a.a-D-Lactose .pyranose e both sweeter than sucrose 12.What is the first step in isomerization of glucose? d.ofabond within the pyranosein e.Oxidation of the carbonyl 13.The lowest energy state for glucose in water at room temperature is c.a pyranose ring in the2Cs chair configuration d.a pyranose ring in a boat configuration e.none of the above@ 14.Which of the following is true of a-D-galactopyranose and B-D-galactopyranose? ers They differ only in configuration about two carbons They have equa ution I hey are bot on products of galactose
FST 100A Midterm 1; October 27, 2004 Page 4 9. Which of the following would NOT contribute calories? a. Reducing sugars b. Sugar alcohols c. Maltodextrins d. Sucrose e. All of the above would contribute calories @ 10. A particular sugar alcohol has a relative sweetness intensity of fsuc,g = 0.5. We would therefore expect that a. 10 g of the sugar alcohol would be as sweet as 10 g of sucrose in a food b. 10 g of the sugar alcohol would be as sweet as 20 g of sucrose in a food c. 10 g of the sugar alcohol would be as sweet as 0.5 g of sucrose in a food d. 20 g of the sugar alcohol would be as sweet as 10 g of sucrose in a food@ e. the sugar alcohol does not have a sweet taste 11. Which of the following is sweeter than sucrose at room temperature? a. α-D-Lactose b. β-D-Fructopyranose@ c. α-D-Maltose d. β-D-Glucopyranose e. Answers a and b are both sweeter than sucrose 12. What is the first step in isomerization of glucose? a. Protonation of a leaving group b. Nucleophilic addition c. Loss of a proton @ d. Cleavage of a C-O bond within the pyranose ring e. Oxidation of the carbonyl 13. The lowest energy state for glucose in water at room temperature is a. as an open chain b. a furanose ring c. a pyranose ring in the 2 C5 chair configuration d. a pyranose ring in a boat configuration e. none of the above@ 14. Which of the following is true of α-D-galactopyranose and β-D-galactopyranose? a. They are enantiomers b. They are anomers @ c. They differ only in configuration about two carbons d. They have equal energies in solution e. They are both oxidation products of galactose

FST 100A Midterm 1;October 27,2004 Page5 15.Hydrolysis of lactose involves .r mrmedate C production of D-glucose and D-fructose d.nucleophilic attack by water@ e.answer b and d are involved 16.Which of the following are true of humectants? a.They have attractive molecular interactions with water b.They are hygroscopic c.They include fructose and glucose d.Th elp to retain moisture in foods e.All of the above are true of humectants@ 17.Which of the following are good reasons to add lemon juice to fruit salad? &T8s8woeRRaoonmgtates To inactivate polyphenoloxidase@ a To aid in lactose digestion Answer a and b only None of the above HC=O 18.In the form shown,the molecule at right can participate as a reactant H- -OH mutarotation HO C-H hydrolysis of a glycosidic linkage Maillard browning HO C-H H- OH e.enzymatic browning 19.Which of the following is true of grape sugar? d.It r found in e.None of the above are true o grape sugar 20.Monosaccharide isomerization reactions are responsible for b.erion of e glucose ut conversion of some glucose to fructose in honey on reaction e. answer a al involve an isomerization reactior
FST 100A Midterm 1; October 27, 2004 Page 5 15. Hydrolysis of lactose involves a. nucleophilic attack by an alcohol b. formation of an enolate ion intermediate c. production of D-glucose and D-fructose d. nucleophilic attack by water @ e. answer b and d are involved 16. Which of the following are true of humectants? a. They have attractive molecular interactions with water b. They are hygroscopic c. They include fructose and glucose d. They help to retain moisture in foods e. All of the above are true of humectants@ 17. Which of the following are good reasons to add lemon juice to fruit salad? a. To inactivate polyphenoloxidase@ b. To slow down Maillard browning rates c. To aid in lactose digestion d. Answer a and b only e. None of the above 18. In the form shown, the molecule at right can participate as a reactant in a. mutarotation b. hydrolysis of a glycosidic linkage c. Maillard browning d. answer a and c@ e. enzymatic browning 19. Which of the following is true of grape sugar? a. It is hygroscopic @ b. It is sweeter than sucrose c. It is a disaccharide d. It is the only sugar found in grapes e. None of the above are true of grape sugar 20. Monosaccharide isomerization reactions are responsible for a. production of high fructose corn syrup b. conversion of some glucose to fructose in honey c. production of invert sugar from sucrose d. all of the above involve an isomerization reaction e. answer a and b involve an isomerization reaction@ C C C C HC OH H OH HO H H HO H CH2OH O