
OCH3 CH:CH2CH2CH3 2 CH3 CH:CH2OCCH2CH3 4 4 CH3
C H3 C H2 C H2 C H3 OCH3 C H3 C H2OCCH2 C H3 O CH3 CH3 2 1 4 4 4 2
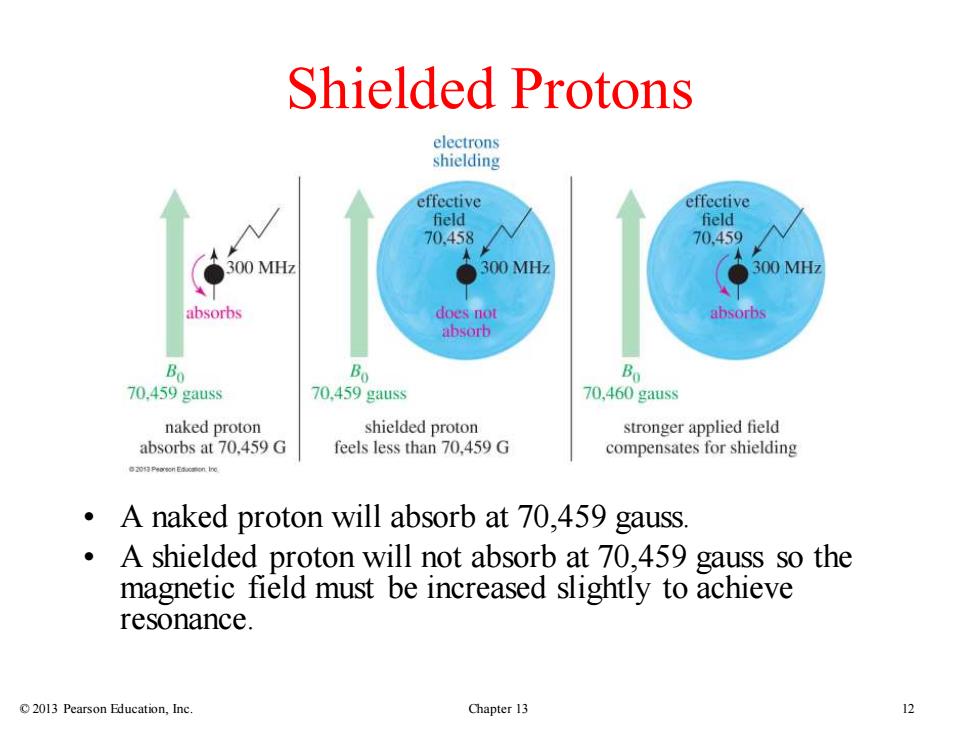
Shielded Protons electrons shielding effective effective field field 70.458 70.459 300 MHz ●300MHz 300MH7 absorbs does not absorbs absorb Bo Bo Bo 70.459 gauss 70.459 gauss 70,460 gauss naked proton shielded proton stronger applied field absorbs at 70,459 G feels less than 70,459 G compensates for shielding A naked proton will absorb at 70,459 gauss A shielded proton will not absorb at 70,459 gauss so the magnetic field must be increased slightly to achieve resonance. 2013 Pearson Education,Inc. Chapter 13
© 2013 Pearson Education, Inc. Chapter 13 12 Shielded Protons • A naked proton will absorb at 70,459 gauss. • A shielded proton will not absorb at 70,459 gauss so the magnetic field must be increased slightly to achieve resonance

CH3Br Since methyl bromide contains only a single type of hydrogen atom,we see only a single peak at 2.7 ppm This is slightly deshielded due to the presence of the Br. No integral since integration gives the relative number of each type and we have only one type. TMS 11 10 6 ppm
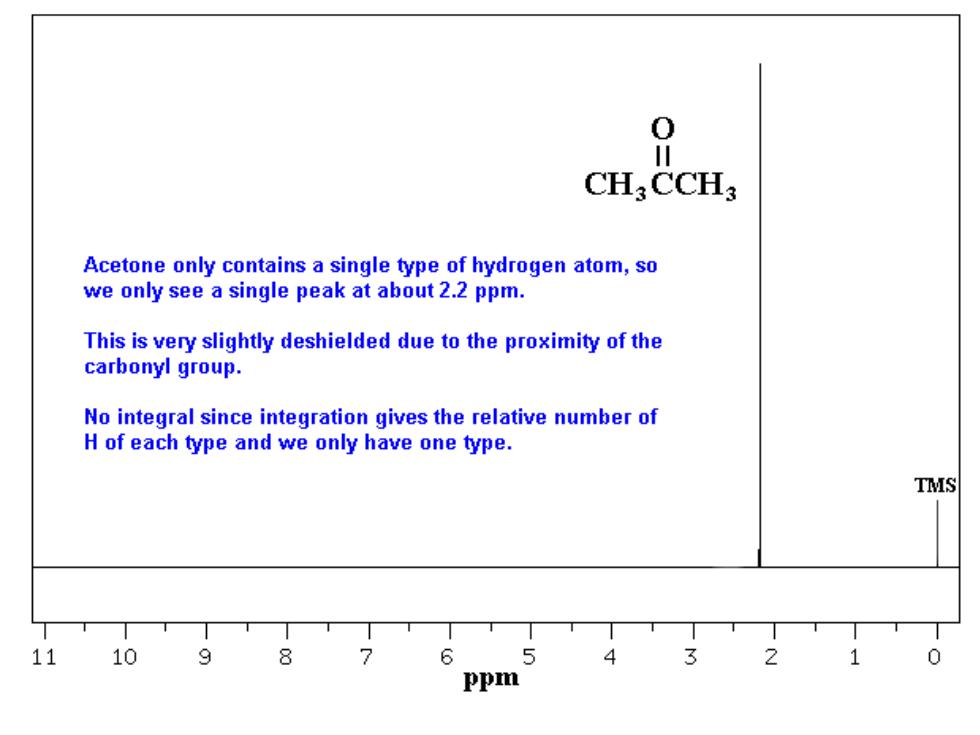
0 CH.CCH3 Acetone only contains a single type of hydrogen atom,so we only see a single peak at about 2.2 ppm. This is very slightly deshielded due to the proximity of the carbonyl group. No integral since integration gives the relative number of H of each type and we only have one type. TMS 11 10 8 5 ppm

CH, Br-CC Since t-butyl bromide contains only a single type of hydrogen atom,we see only a single peak at 1.8 ppm. Since the Br atom is further away from the H atoms than methyl bromide,there is less deshielding than in methyl bromide. No integral since integration gives the relative number of each type,and we have only one type. 10 9 8 5 0 Ppm