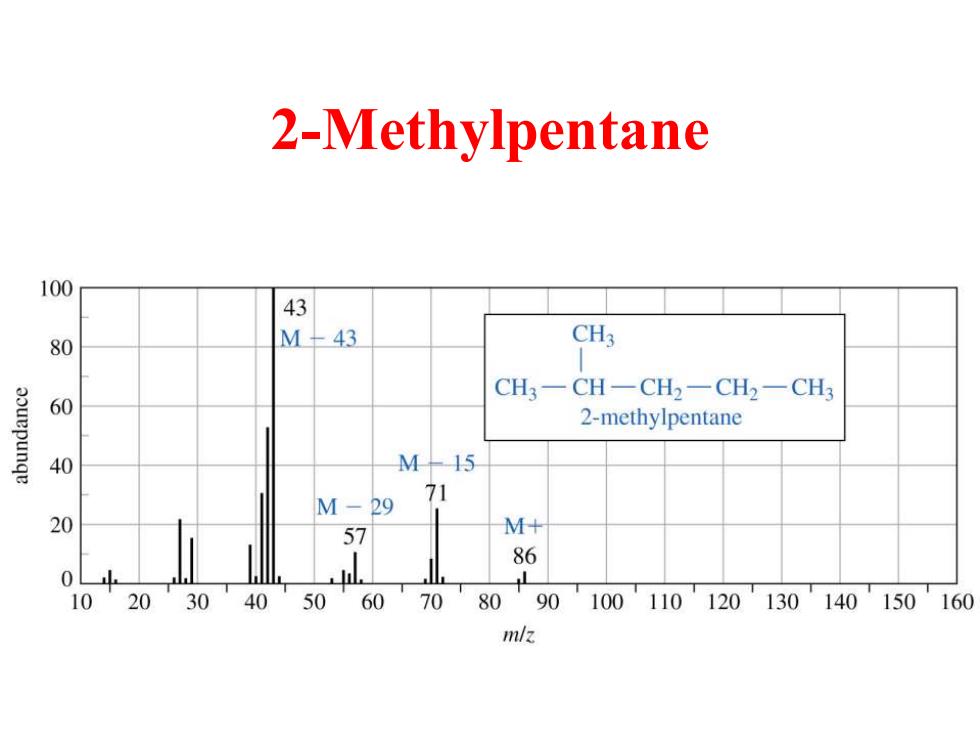
2-Methylpentane 100 43 80 M-43 CH3 CH3一CH-CH2一CH2-CH 60 2-methylpentane 40 M-45 M-29 71 20 57 M+ 86 0 10 20 30 40 50 60 70 80 90100110120130140150160 m/z
2-Methylpentane

Effect of Branching in Hydrocarbons 43 57 100 n-Hexadecane 717 :26 50 M 9911131 1417155 18311977 2267 0 TTTTTT 50 100 m/ 150 200 169141 43 100 57:85 57 5-Methylpentadecane 85 C16H34 50 Mol.Wt:226 71 99 168 M 12771417 21112267 0 50 100 / 150 200
Effect of Branching in Hydrocarbons

Isotopes Mass spectrometers are capable of separating and detecting individual ions even those that only differ by a single atomic mass unit. As a result molecules containing different isotopes can be distinguished. This is most apparent when atoms such as bromine or chlorine are present(79Br:81Br,intensity 1:1 and 35CI: 37Cl,intensity 3:1)where peaks at "M"and "M+2"are obtained. The intensity ratios in the isotope patterns are due to the natural abundance of the isotopes. "M+1"peaks are seen due the the presence of 13C in the sample
Isotopes • Mass spectrometers are capable of separating and detecting individual ions even those that only differ by a single atomic mass unit. • As a result molecules containing different isotopes can be distinguished. • This is most apparent when atoms such as bromine or chlorine are present (79Br : 81Br, intensity 1:1 and 35Cl : 37Cl, intensity 3:1) where peaks at "M" and "M+2" are obtained. • The intensity ratios in the isotope patterns are due to the natural abundance of the isotopes. • "M+1" peaks are seen due the the presence of 13C in the sample
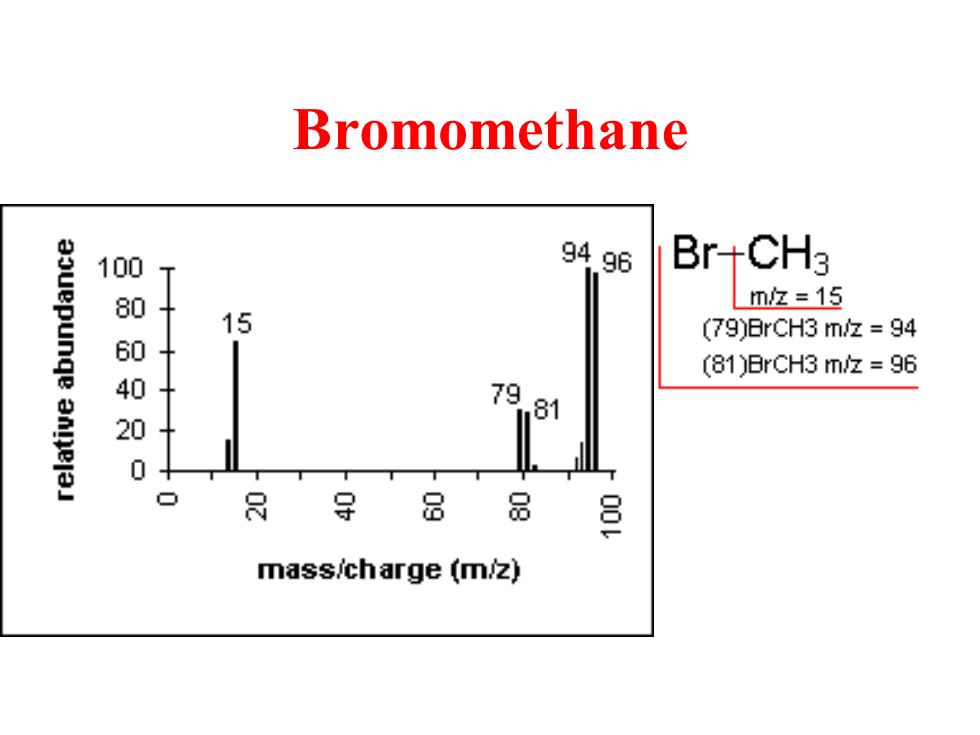
Bromomethane aouepunge 100 9496 BrCH3 8 mz=15 15 (79)BrCH3 m/z =94 (81)BrCH3 m/z =96 40 79 anielaJ 81 0 8导 8 品 三 mass/charge (m/z)
Bromomethane

1-Bromopropane 100 80 CH3-CH2-CH2-Br s5uepunqe 60 M 122(C3H27Br) M+2 40 124(C3H,8Br) 20 010420"30405060708090100i0120130140150160 T m/2
1-Bromopropane