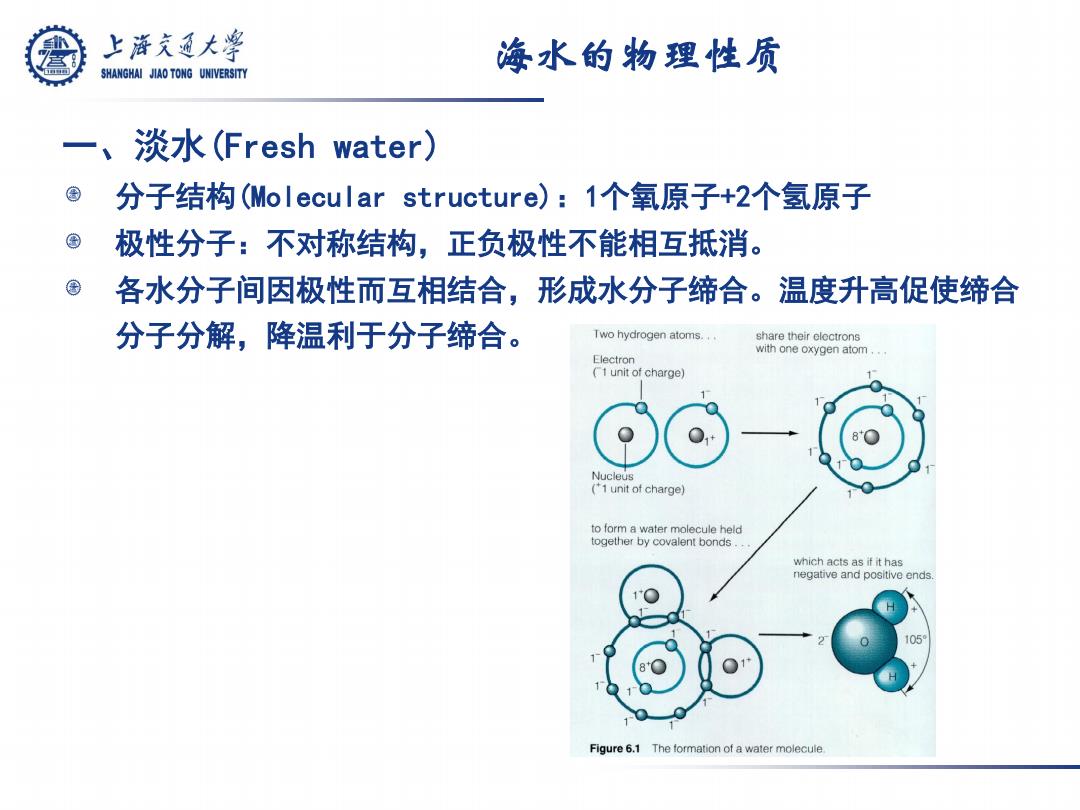
上游充通大兽 海水的物理性质 SHANGHAI JIAO TONG UNIVERSITY 一、淡水(Fresh water) 分子结构(Molecular structure)):1个氧原子+2个氢原子 © 极性分子:不对称结构,正负极性不能相互抵消。 各水分子间因极性而互相结合,形成水分子缔合。温度升高促使缔合 分子分解,降温利于分子缔合。 Two hydrogen atoms.. share their elcctrons with one oxygen atom Electron (1 unit of charge) Nucleus (1 unit of charge) to form a water molecule held together by covalent bonds.. which acts as if it has negative and positive ends Figure 6.1 The formation of a water molecule
海水的物理性质 一、淡水(Fresh water) 分子结构(Molecular structure):1个氧原子+2个氢原子 极性分子:不对称结构,正负极性不能相互抵消。 各水分子间因极性而互相结合,形成水分子缔合。温度升高促使缔合 分子分解,降温利于分子缔合
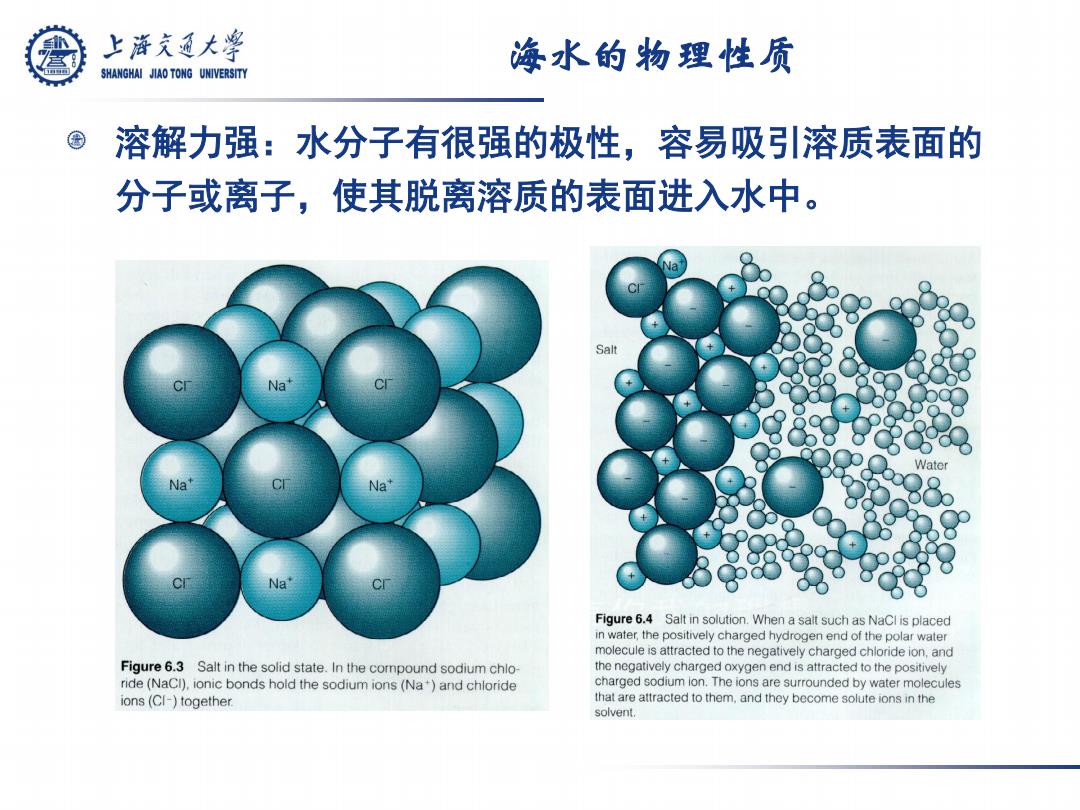
上游究通大学 海水的物理性质 SHANGHAI JIAO TONG UNIVERSITY 溶解力强:水分子有很强的极性,容易吸引溶质表面的 分子或离子,使其脱离溶质的表面进入水中。 Salt Na Na Figure 6.4 Salt in solution.When a salt such as NaCl is placed in water,the positively charged hydrogen end of the polar water molecule is attracted to the negatively charged chloride ion,and Figure 6.3 Salt in the solid state.In the compound sodium chlo- the negatively charged oxygen end is attracted to the positively ride(NaCI),ionic bonds hold the sodium ions (Na)and chloride charged sodium ion.The ions are surrounded by water molecules ions(CI-)together. that are attracted to them,and they become solute ions in the solvent
海水的物理性质 溶解力强:水分子有很强的极性,容易吸引溶质表面的 分子或离子,使其脱离溶质的表面进入水中
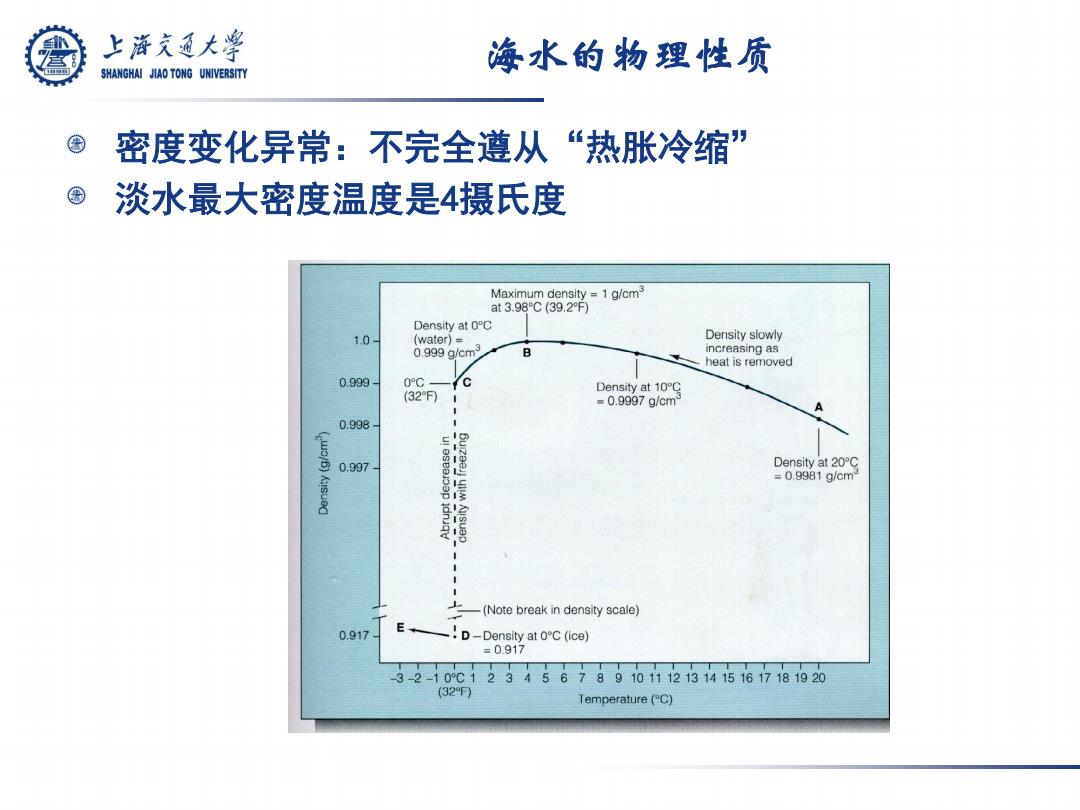
上游究通大学 海水的物理性质 SHANGHAI JIAO TONG UNIVERSITY 密度变化异常:不完全遵从“热胀冷缩” 淡水最大密度温度是4摄氏度 Maximum density=1 g/cm3 at3.98℃(39.2F) Density at0℃ 1.0 water)= Density slowly 0,999g/cm3 B increasing as heat is removed 0.999 0°C- Density at 10C (32F) =0.9997g/cm 0.998 CIO 0.997 Density at 20C =0.9981g/cm (Note break in density scale) 0.917 E -!D-Density at OC(ice) =0.917 3-2-10°C1234567891011121314151617181920 (32F) Temperature (C)
海水的物理性质 密度变化异常:不完全遵从“热胀冷缩” 淡水最大密度温度是4摄氏度

上游充通大兽 海水的物理性质 SHANGHAI JIAO TONG UNIVERSITY 水的热性质特殊: 沸点(boiling point)和融点(melting point)、比热 (specific heat)、蒸发潜热(latent heat of vapor ization)等热性质比氧的同族化合物高(理论上的沸 点为-80℃,实际100℃) ©汽化等需消耗较多能量
海水的物理性质 水的热性质特殊: 沸点(boiling point)和融点(melting point)、比热 (specific heat)、蒸发潜热(latent heat of vaporization)等热性质比氧的同族化合物高(理论上的沸 点为-80℃,实际100℃) 汽化等需消耗较多能量

上游充通大兽 海水的物理性质 SHANGHAI JIAO TONG UNIVERSITY 二、海水的热力学性质(Energetic property of sea water) 1)热容(heat capacity)、比热容(specific heat capacity) 热容:海水温度升高1K(或1C)所吸收的热量(J/K,J/C) 比热容:单位质量海水的热容。J/(Kkg),J/(kgC) ©海水的比热约3.89×103J/(kg℃) 空气的比热为1×103J/(kgC) ©海水热容量:空气热容量≈3100:1
海水的物理性质 二、海水的热力学性质(Energetic property of sea water) 1) 热容(heat capacity)、比热容(specific heat capacity) 热容:海水温度升高1K(或1℃)所吸收的热量(J/K,J/℃) 比热容:单位质量海水的热容。J/(K kg),J/(kg ℃) 海水的比热约3.89×103 J/(kg ℃) 空气的比热为1×103 J/(kg ℃) 海水热容量:空气热容量≈3100:1