
含有氢化吡喃螺环的大环内酯 In 1993 three independant research groups reported a new class of remarkably cytotoxic marine macrolides,the spongipyrans,from marine sponges for the first time. The research was guided by bioassays and led to the identification and structure determination of spongistatins 1-9 (Pettit et al.)from Spongia sp.and Spirastrella spinispirulifera,cinachyrolide A (Fusetani et al.)from Cinachyra,and altohyrtins A-C as well as 5- desacetylaltohyrtin A (Kitagawa et al.)from Hyrtios
• In 1993 three independant research groups reported a new class of remarkably cytotoxic marine macrolides, the spongipyrans, from marine sponges for the first time. • The research was guided by bioassays and led to the identification and structure determination of spongistatins 1-9 (Pettit et al.) from Spongia sp. and Spirastrella spinispirulifera, cinachyrolide A (Fusetani et al.) from Ci n a c h y r a , a n d a lt o h y r ti n s A - C a s w e l l a s 5 - desacetylaltohyrtin A (Kitagawa et al.) from Hyrtios
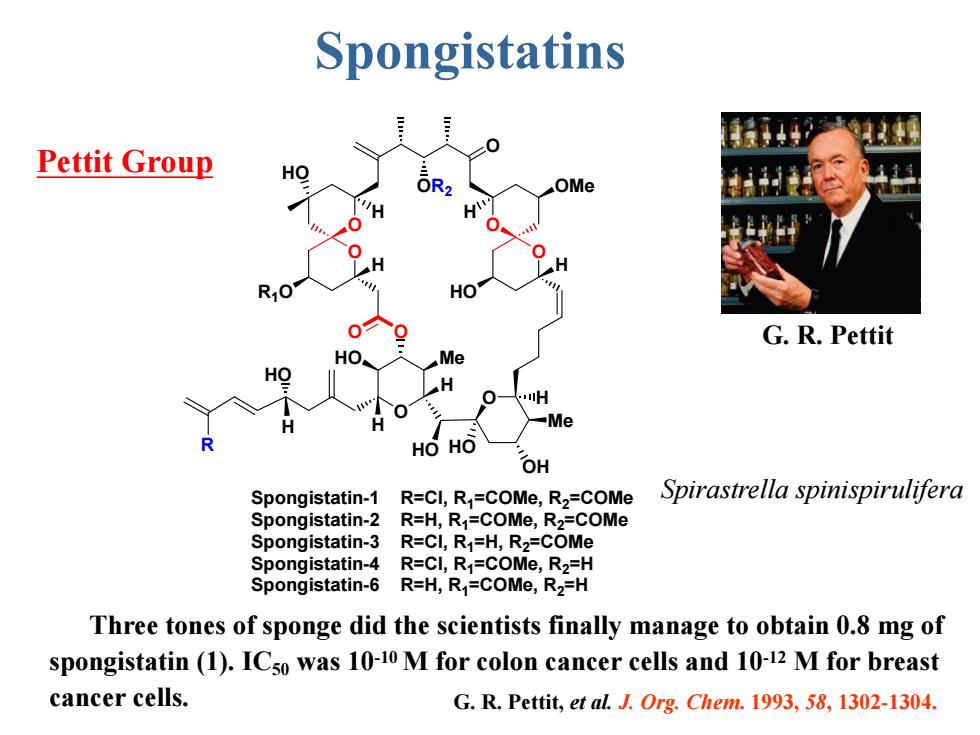
Spongistatins Pettit Group HO OMe H HO G.R.Pettit HO Me H H -Me HOHO OH Spongistatin-1 R=CI,R=COMe,R2=COMe Spirastrella spinispirulifera Spongistatin-2 R=H,R1=COMe,R2=COMe Spongistatin-3 R=CI,R1=H,R2=COMe Spongistatin-4 R=CI,R=COMe,R2=H Spongistatin-6 R=H,R=COMe,R2=H Three tones of sponge did the scientists finally manage to obtain 0.8 mg of spongistatin (1).ICso was 10-10 M for colon cancer cells and 10-12 M for breast cancer cells. G.R.Pettit,et al.J.Org.Chem.1993,58,1302-1304
G. R. Pettit, et al. J. Org. Chem. 1993, 58, 1302-1304. Spongistatins Pettit Group O O O Me OH O O O OR2 HO O O HO Me O HO H R HO R1O HO OMe H H H HO H H H H Spongistatin-1 R=Cl, R1=COMe, R2=COMe Spongistatin-2 R=H, R1=COMe, R2=COMe Spongistatin-3 R=Cl, R1=H, R2=COMe Spongistatin-4 R=Cl, R1=COMe, R2=H Spongistatin-6 R=H, R1=COMe, R2=H G. R. Pettit Three tones of sponge did the scientists finally manage to obtain 0.8 mg of spongistatin (1). IC50 was 10-10 М for colon cancer cells and 10-12 М for breast cancer cells. Spirastrella spinispirulifera
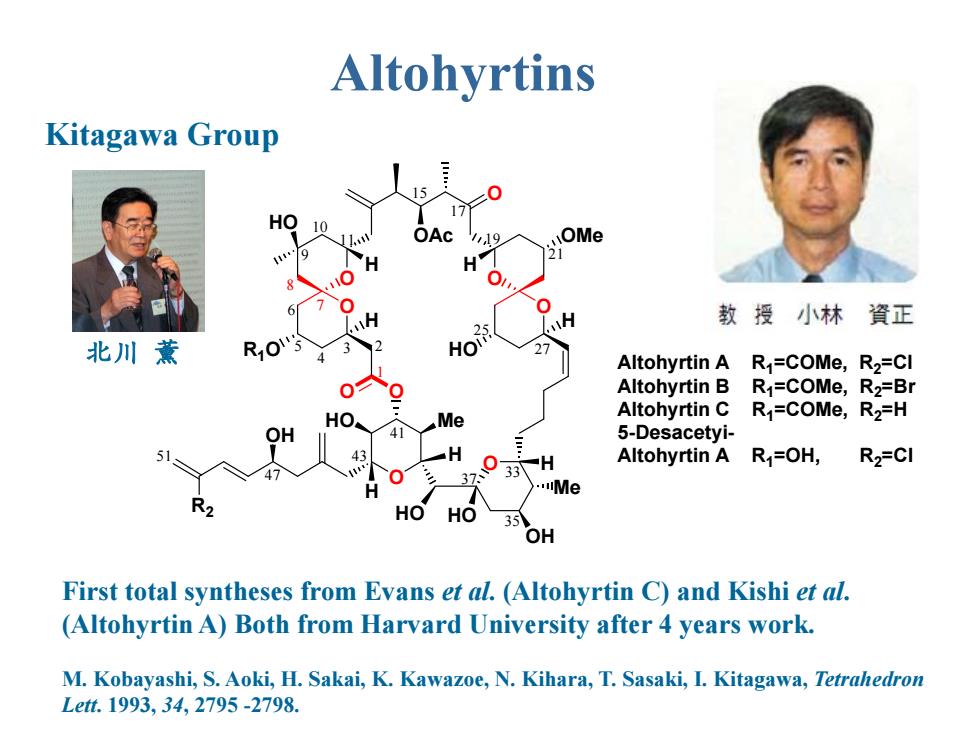
Altohyrtins Kitagawa Group 17 HO 10 OAc 119 OMe 9 H 3 6 0 H H 教授小林資正 25 北川薰 2 Altohyrtin A R1=COMe,R2=CI Altohyrtin B R1=COMe,R2=Br HO Me Altohyrtin C R1=COMe,R2=H OH 5-Desacetyi- 51 43 H H Altohyrtin A R=OH, R2=CI 0 37 33 Me R2 HO HO 35 OH First total syntheses from Evans et al.(Altohyrtin C)and Kishi et al. (Altohyrtin A)Both from Harvard University after 4 years work. M.Kobayashi,S.Aoki,H.Sakai,K.Kawazoe,N.Kihara,T.Sasaki,I.Kitagawa,Tetraledron Lett.1993,34,2795-2798
M. Kobayashi, S. Aoki, H. Sakai, K. Kawazoe, N. Kihara, T. Sasaki, I. Kitagawa, Tetrahedron Lett. 1993, 34, 2795 -2798. First total syntheses from Evans et al. (Altohyrtin C) and Kishi et al. (Altohyrtin A) Both from Harvard University after 4 years work. Kitagawa Group O O O Me OH O O O OAc HO O O HO Me O HO H OH R1O HO OMe H H H HO H H H Altohyrtin A R1=COMe, R2=Cl Altohyrtin B R1=COMe, R2=Br Altohyrtin C R1=COMe, R2=H 5-Desacetyi- Altohyrtin A R1=OH, R2=Cl R2 3 2 4 5 6 7 8 9 10 11 15 17 19 21 25 27 33 35 37 41 43 47 51 1 Altohyrtins 北川 薰
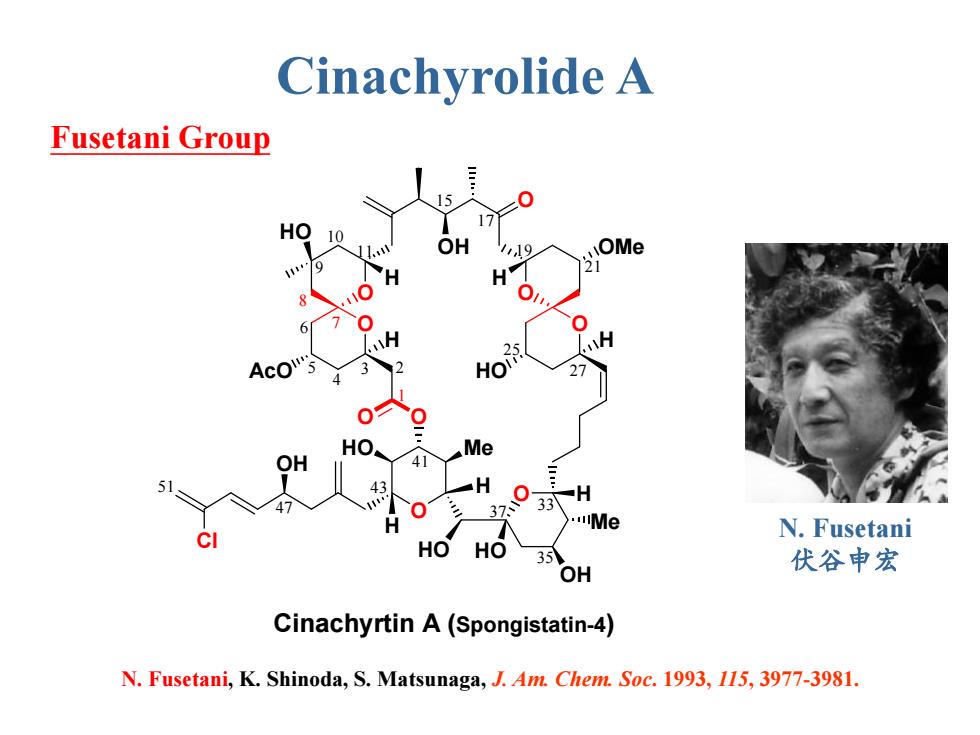
Cinachyrolide A Fusetani Group 17 HO 10 OH 19 OMe 21 H 6 25 HO 3 Me 41 51 X H 0 37 33引H Me N.Fusetani HOHO 35 OH 伏谷申宏 Cinachyrtin A(Spongistatin-4) N.Fusetani,K.Shinoda,S.Matsunaga,J.Am.Chem.Soc.1993,115,3977-3981
N. Fusetani, K. Shinoda, S. Matsunaga, J. Am. Chem. Soc. 1993, 115, 3977-3981. Fusetani Group O O O Me OH O O O OH HO O O HO Me O HO H OH AcO HO OMe H H H HO H H H Cl 3 2 4 5 6 7 8 9 10 11 15 17 19 21 25 27 33 35 37 41 43 47 51 1 Cinachyrtin A (Spongistatin-4) Cinachyrolide A N. Fusetani 伏谷申宏
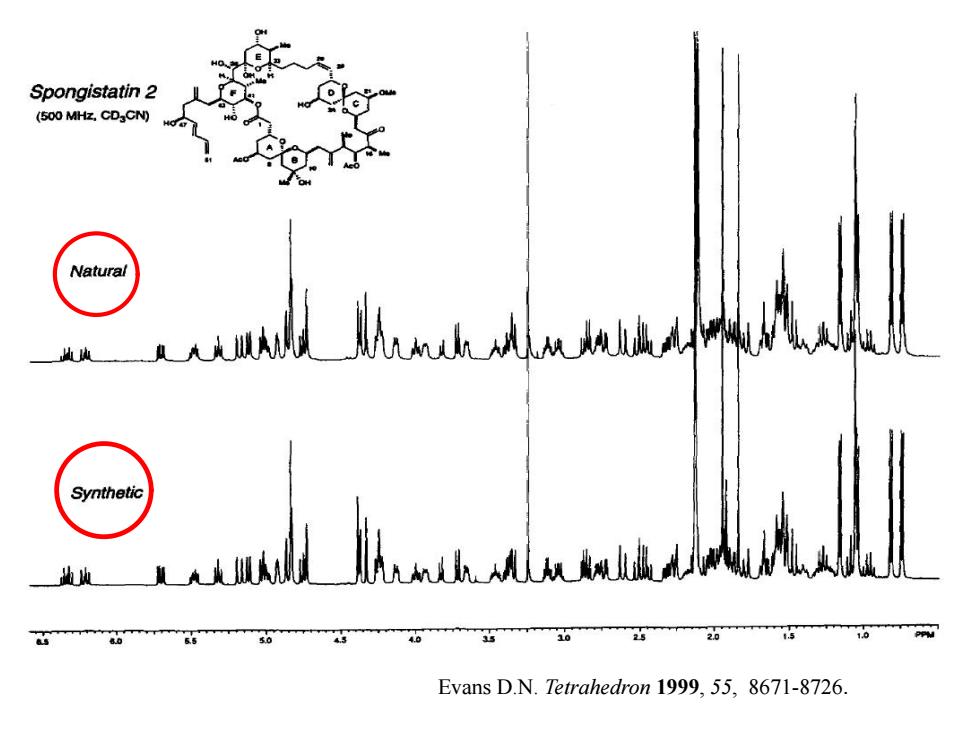
Spongistatin 2 (500 MHz,CDaCN) Natural 人46 Synthetic 20 Evans D.N.Tetrahedron 1999,55,8671-8726
Evans D.N. Tetrahedron 1999, 55, 8671-8726