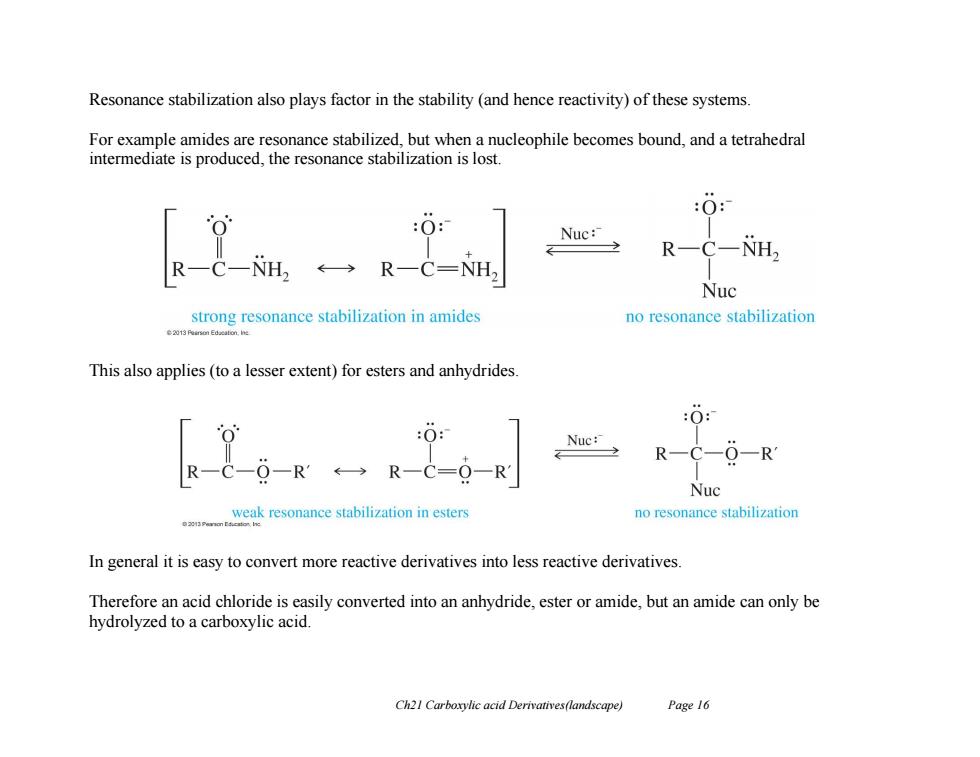
Resonance stabilization also plays factor in the stability (and hence reactivity)of these systems For example amides are resonance stabilized,but when a nucleophile becomes bound,and a tetrahedral intermediate is produced,the resonance stabilization is lost. :0: :0 Nuc: R-C-NH, R-C-NH2 ←→R-C=NH2 Nuc strong resonance stabilization in amides no resonance stabilization This also applies(to a lesser extent)for esters and anhydrides. :0: Nuc: R-C-O-R R一C O-R ←→R一C=O-R1 Nuc weak resonance stabilization in esters no resonance stabilization In general it is easy to convert more reactive derivatives into less reactive derivatives Therefore an acid chloride is easily converted into an anhydride,ester or amide,but an amide can only be hydrolyzed to a carboxylic acid. Ch21 Carboxylic acid Derivatives(landscape) Page 16
Ch21 Carboxylic acid Derivatives(landscape) Page 16 Resonance stabilization also plays factor in the stability (and hence reactivity) of these systems. For example amides are resonance stabilized, but when a nucleophile becomes bound, and a tetrahedral intermediate is produced, the resonance stabilization is lost. This also applies (to a lesser extent) for esters and anhydrides. In general it is easy to convert more reactive derivatives into less reactive derivatives. Therefore an acid chloride is easily converted into an anhydride, ester or amide, but an amide can only be hydrolyzed to a carboxylic acid
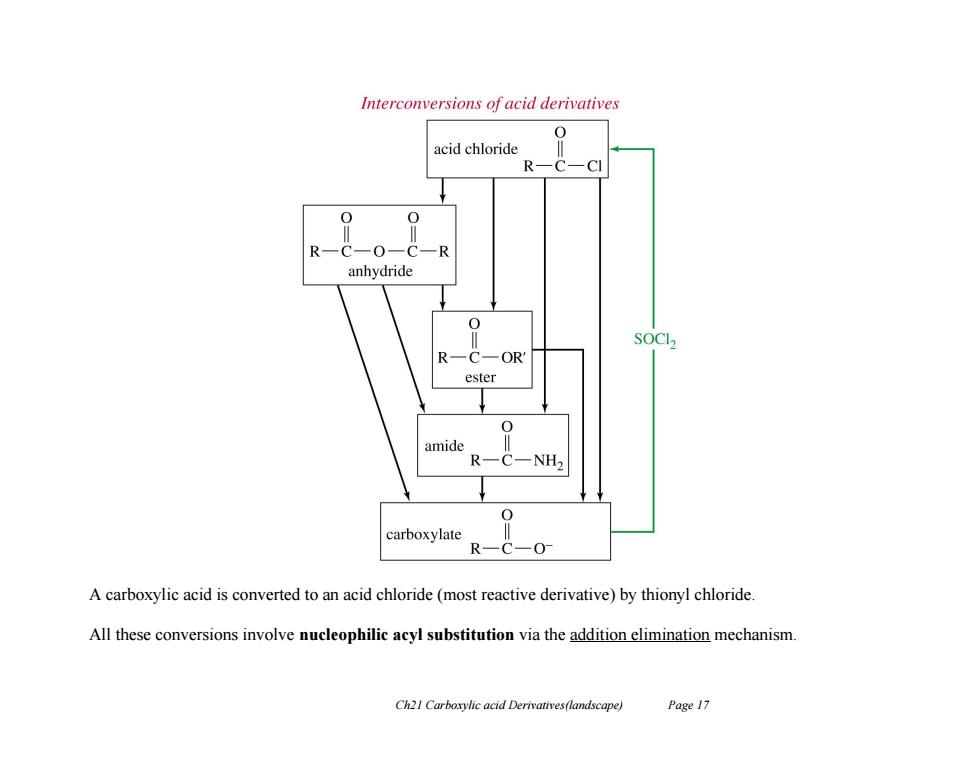
Interconversions of acid derivatives 0 acid chloride R-C-CI C-0-C-R anhydride R 一OR ester amide R NH2 carboxylate R-C -0 A carboxylic acid is converted to an acid chloride (most reactive derivative)by thionyl chloride. All these conversions involve nucleophilic acyl substitution via the addition elimination mechanism. Ch21 Carboxylic acid Derivatives(landscape) Page 17
Ch21 Carboxylic acid Derivatives(landscape) Page 17 A carboxylic acid is converted to an acid chloride (most reactive derivative) by thionyl chloride. All these conversions involve nucleophilic acyl substitution via the addition elimination mechanism