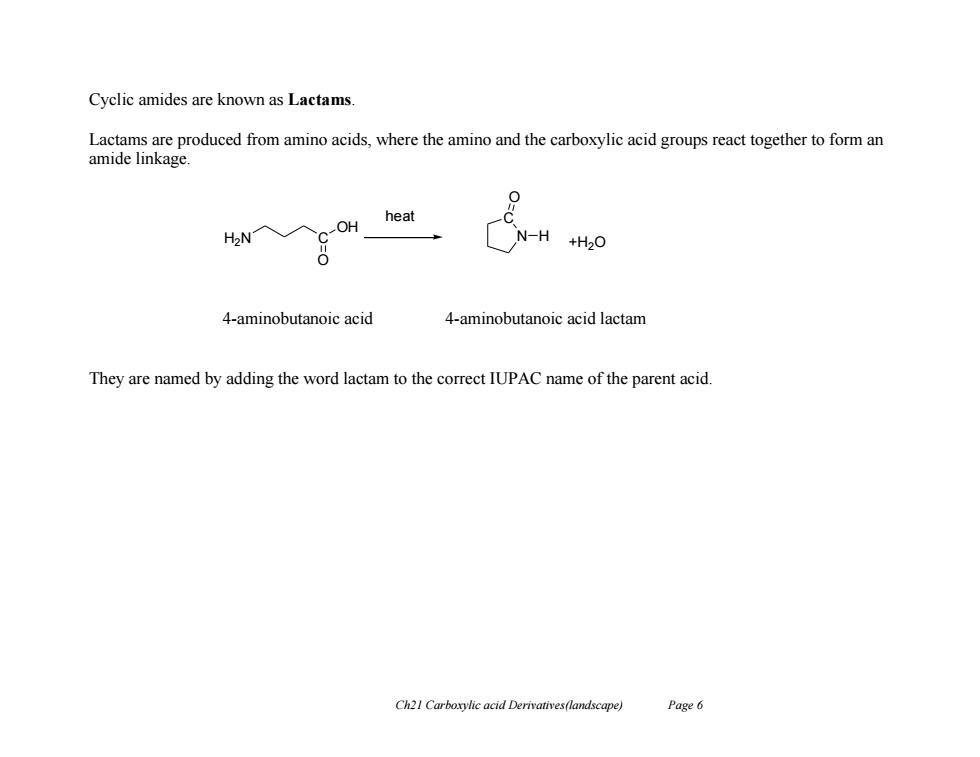
Cyclic amides are known as Lactams Lactams are produced from amino acids,where the amino and the carboxylic acid groups react together to form an amide linkage. 0 heat OH N-H 4-aminobutanoic acid 4-aminobutanoic acid lactam They are named by adding the word lactam to the correct IUPAC name of the parent acid. Ch21 Carboxylic acid Derivatives(landscape) Page 6
Ch21 Carboxylic acid Derivatives(landscape) Page 6 Cyclic amides are known as Lactams. Lactams are produced from amino acids, where the amino and the carboxylic acid groups react together to form an amide linkage. 4-aminobutanoic acid 4-aminobutanoic acid lactam They are named by adding the word lactam to the correct IUPAC name of the parent acid. H2N C OH O N C O H +H2O heat
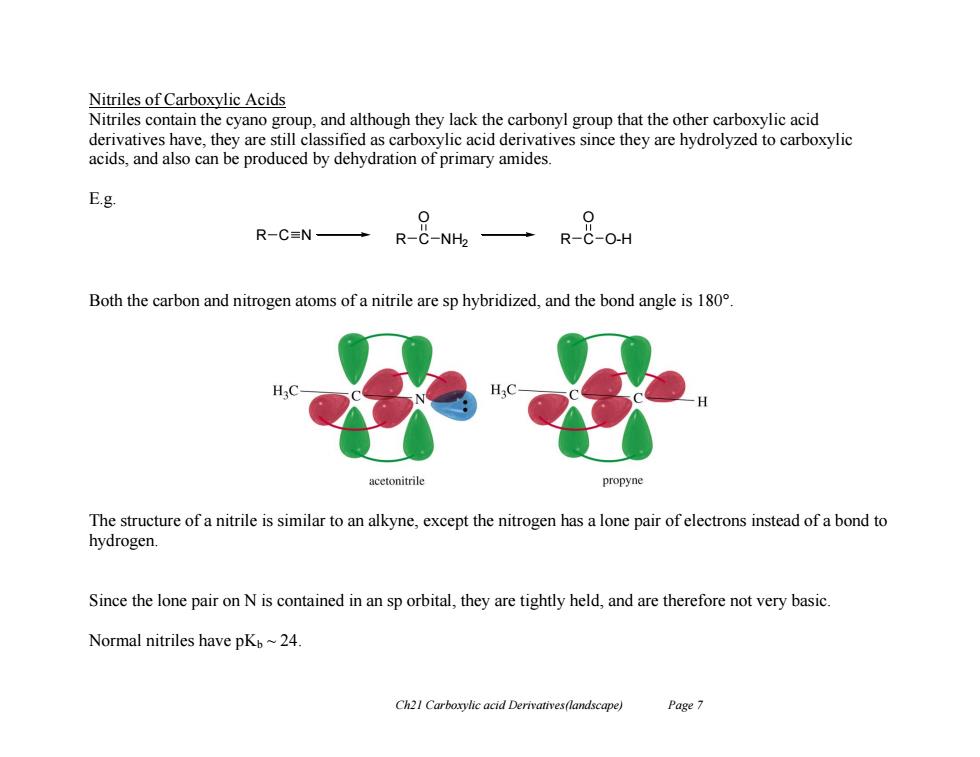
Nitriles of Carboxylic Acids Nitriles contain the cyano group,and although they lack the carbonyl group that the other carboxylic acid derivatives have,they are still classified as carboxylic acid derivatives since they are hydrolyzed to carboxylic acids,and also can be produced by dehydration of primary amides. E.g. 0 R-CN→ R-C-NH2 →R-&-OH Both the carbon and nitrogen atoms of a nitrile are sp hybridized,and the bond angle is 180. H.C acetonitrile propyne The structure of a nitrile is similar to an alkyne,except the nitrogen has a lone pair of electrons instead of a bond to hydrogen. Since the lone pair on N is contained in an sp orbital,they are tightly held,and are therefore not very basic. Normal nitriles have pKb~24. Ch21 Carboxylic acid Derivatives(landscape) Page 7
Ch21 Carboxylic acid Derivatives(landscape) Page 7 Nitriles of Carboxylic Acids Nitriles contain the cyano group, and although they lack the carbonyl group that the other carboxylic acid derivatives have, they are still classified as carboxylic acid derivatives since they are hydrolyzed to carboxylic acids, and also can be produced by dehydration of primary amides. E.g. Both the carbon and nitrogen atoms of a nitrile are sp hybridized, and the bond angle is 180°. The structure of a nitrile is similar to an alkyne, except the nitrogen has a lone pair of electrons instead of a bond to hydrogen. Since the lone pair on N is contained in an sp orbital, they are tightly held, and are therefore not very basic. Normal nitriles have pKb ~ 24. R C N R C O NH2 R C O O-H
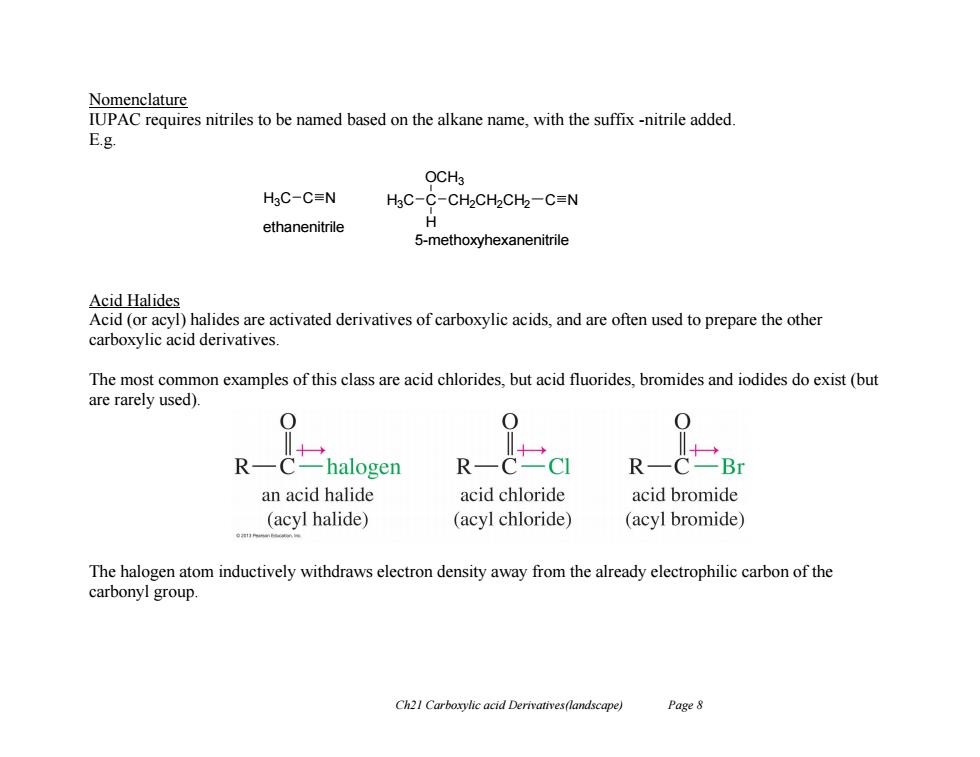
Nomenclature IUPAC requires nitriles to be named based on the alkane name,with the suffix -nitrile added. E.g. OCH3 H3C-C=N HgC-C-CH2CH2CH2-C=N ethanenitrile H 5-methoxyhexanenitrile Acid Halides Acid(or acyl)halides are activated derivatives of carboxylic acids,and are often used to prepare the other carboxylic acid derivatives. The most common examples of this class are acid chlorides,but acid fluorides,bromides and iodides do exist(but are rarely used). R halogen R R C -Br an acid halide acid chloride acid bromide (acyl halide) (acyl chloride) (acyl bromide) The halogen atom inductively withdraws electron density away from the already electrophilic carbon of the carbonyl group. Ch21 Carboxylic acid Derivatives(landscape) Page 8
Ch21 Carboxylic acid Derivatives(landscape) Page 8 Nomenclature IUPAC requires nitriles to be named based on the alkane name, with the suffix -nitrile added. E.g. Acid Halides Acid (or acyl) halides are activated derivatives of carboxylic acids, and are often used to prepare the other carboxylic acid derivatives. The most common examples of this class are acid chlorides, but acid fluorides, bromides and iodides do exist (but are rarely used). The halogen atom inductively withdraws electron density away from the already electrophilic carbon of the carbonyl group. H3C C N ethanenitrile 5-methoxyhexanenitrile H3C C OCH3 H CH2CH2CH2 C N
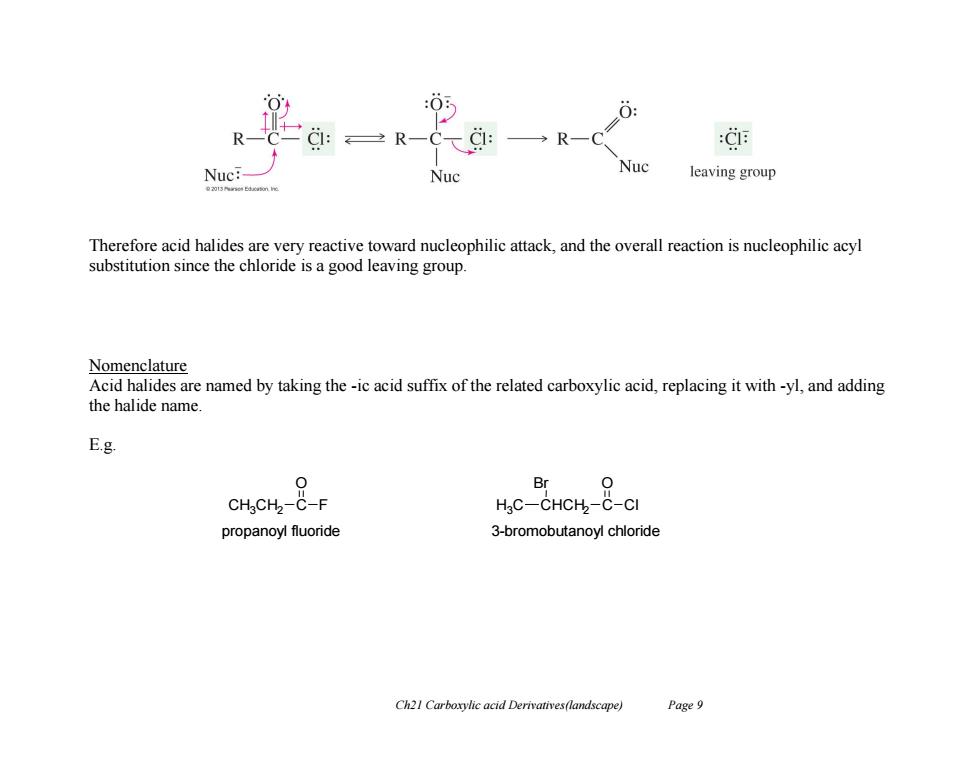
R一C R-Ca :C Nuc: Nuc Nuc leaving group Therefore acid halides are very reactive toward nucleophilic attack,and the overall reaction is nucleophilic acyl substitution since the chloride is a good leaving group. Nomenclature Acid halides are named by taking the-ic acid suffix of the related carboxylic acid,replacing it with-yl,and adding the halide name. E.g. 0 Br CHgCH2-C-F H C-CHCH-C-CI propanoyl fluoride 3-bromobutanoyl chloride Ch21 Carboxylic acid Derivatives(landscape) Page 9
Ch21 Carboxylic acid Derivatives(landscape) Page 9 Therefore acid halides are very reactive toward nucleophilic attack, and the overall reaction is nucleophilic acyl substitution since the chloride is a good leaving group. Nomenclature Acid halides are named by taking the -ic acid suffix of the related carboxylic acid, replacing it with -yl, and adding the halide name. E.g. CH3CH2 C O F CHCH2 C O Cl Br H3C propanoyl fluoride 3-bromobutanoyl chloride

Anhydrides of Carboxylic Acids The word anhydride literally means without water,and an acid anhydride is the combination of two molecules of carboxylic acid with the elimination of one molecule of water 0 0 R-C-O-H H-O-C-R R C-O-C-R' +H2O Anhydrides are also considered as activated forms of carboxylic acids,although anhydrides are not as reactive as acid halides The anhydride group also inductively withdraws electron density from the carbonyl carbon,and the carboxylate anion serves as a good leaving group. RCO Q-C-R R-C :0-C-R Nuc: carboxylate leaving group Half of the anhydride is'lost'as the leaving group,and if the carboxylic acid is very precious(expensive or limited quantity)then this is an undesirable way of making an activated carboxylic acid,and the acid chloride route would be more desirable. Ch21 Carboxylie acid Derivatives(landscape) Page 10
Ch21 Carboxylic acid Derivatives(landscape) Page 10 Anhydrides of Carboxylic Acids The word anhydride literally means without water, and an acid anhydride is the combination of two molecules of carboxylic acid with the elimination of one molecule of water. Anhydrides are also considered as activated forms of carboxylic acids, although anhydrides are not as reactive as acid halides. The anhydride group also inductively withdraws electron density from the carbonyl carbon, and the carboxylate anion serves as a good leaving group. Half of the anhydride is 'lost' as the leaving group, and if the carboxylic acid is very precious (expensive or limited quantity) then this is an undesirable way of making an activated carboxylic acid, and the acid chloride route would be more desirable. R C O O-H C R O H-O R C O O C O R' + H2O