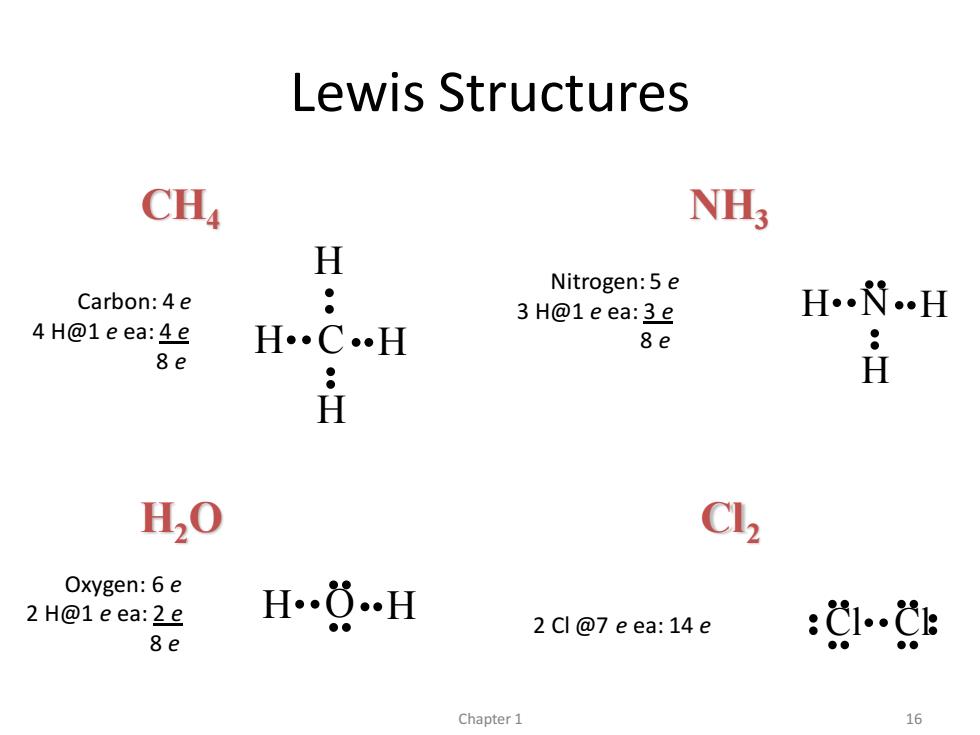
Lewis Structures CHa NH; H Nitrogen:5 e Carbon:4e 3 H@1e ea:3e …H 4H@1eea:4e H· •H 8e 8e H H20 Cl2 Oxygen:6 e 2 H@1e ea:2e …H 2 Cl@7 eea:14e 8e 1… Chapter 1 16
CH4 NH3 H2O Cl2 Lewis Structures C H H H H N H H H H O H Cl Cl Carbon: 4 e 4 H@1 e ea: 4 e 8 e Nitrogen: 5 e 3 H@1 e ea: 3 e 8 e Oxygen: 6 e 2 H@1 e ea: 2 e 8 e 2 Cl @7 e ea: 14 e Chapter 1 16
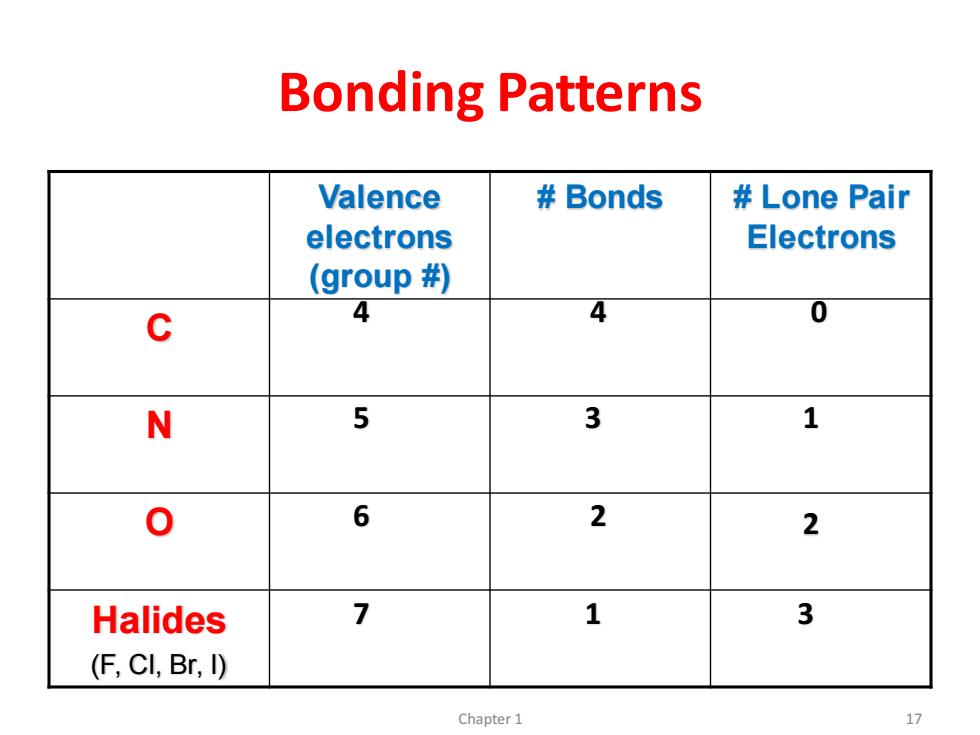
Bonding Patterns Valence Bonds Lone Pair electrons Electrons (group # C 4 4 0 N 5 3 1 0 6 2 2 Halides 7 1 3 (F,CI,Br,I) Chapter 1 17
Bonding Patterns Valence electrons (group #) # Bonds # Lone Pair Electrons C N O Halides (F, Cl, Br, I) 4 4 0 5 3 1 6 2 2 7 1 3 Chapter 1 17
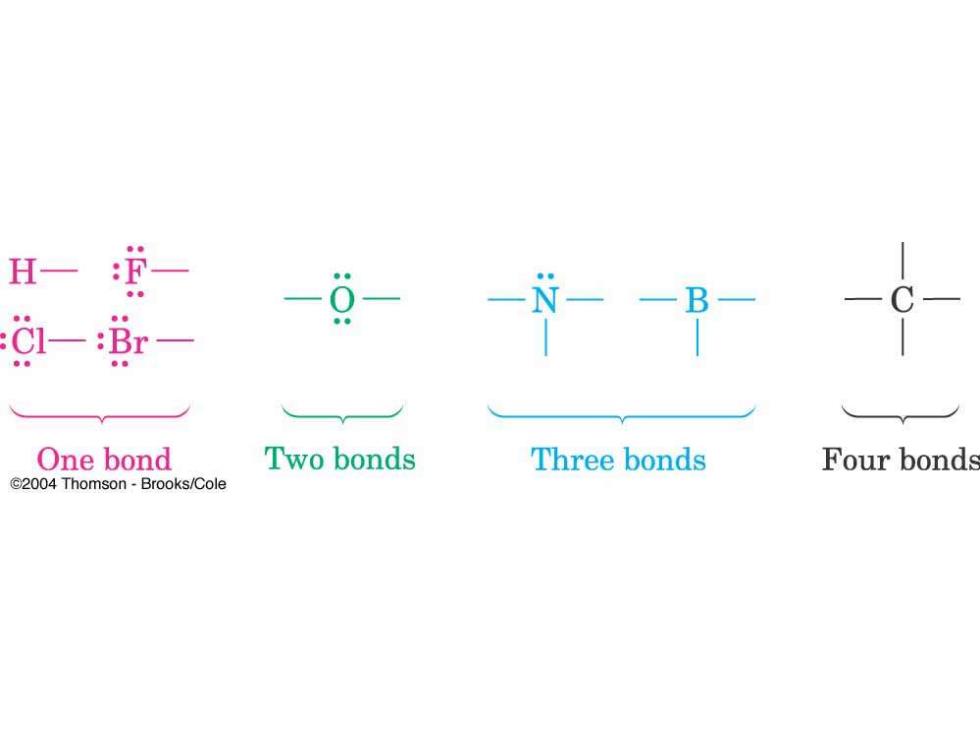
H一 -9- Cl-:Br One bond Two bonds Three bonds Four bonds C2004 Thomson-Brooks/Cole
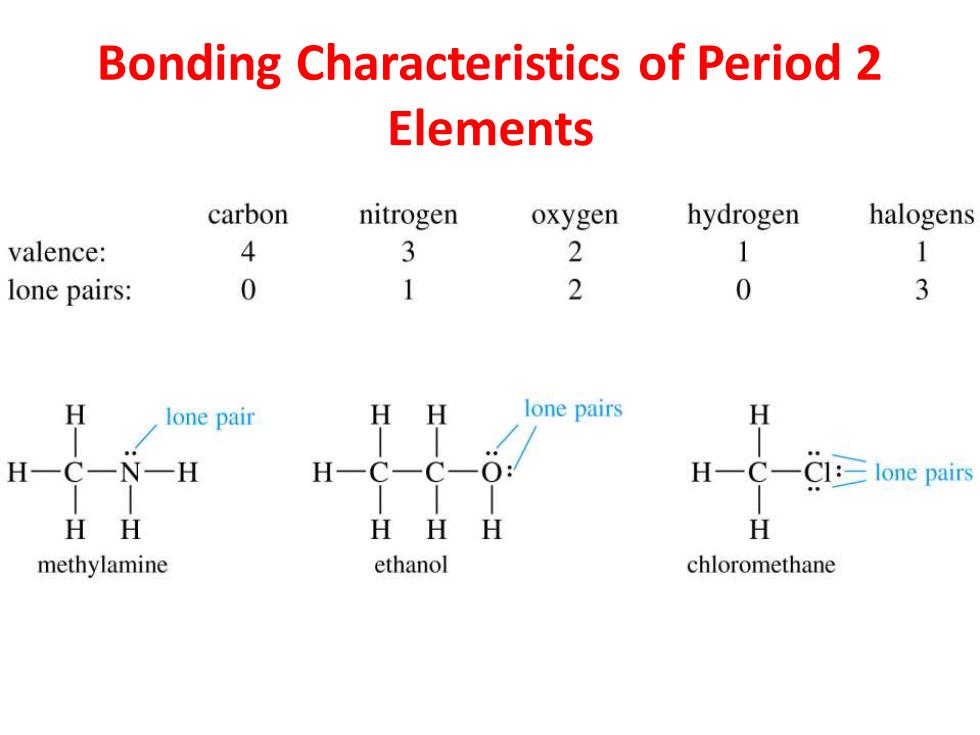
Bonding Characteristics of Period 2 Elements carbon nitrogen oxygen hydrogen halogens valence: 4 3 2 1 1 lone pairs: 0 1 2 0 3 H lone pair H H lone pairs H H一CN一H H一 C- C-0: H-C-Cl:lone pairs HH HH H H methylamine ethanol chloromethane
Bonding Characteristics of Period 2 Elements

HINT Lewis structures are the way we write organic chemistry. Learning now to draw them quickly and correctly will help you throughout this course. Chapter1 20
Lewis structures are the way we write organic chemistry. Learning now to draw them quickly and correctly will help you throughout this course. Chapter 1 20