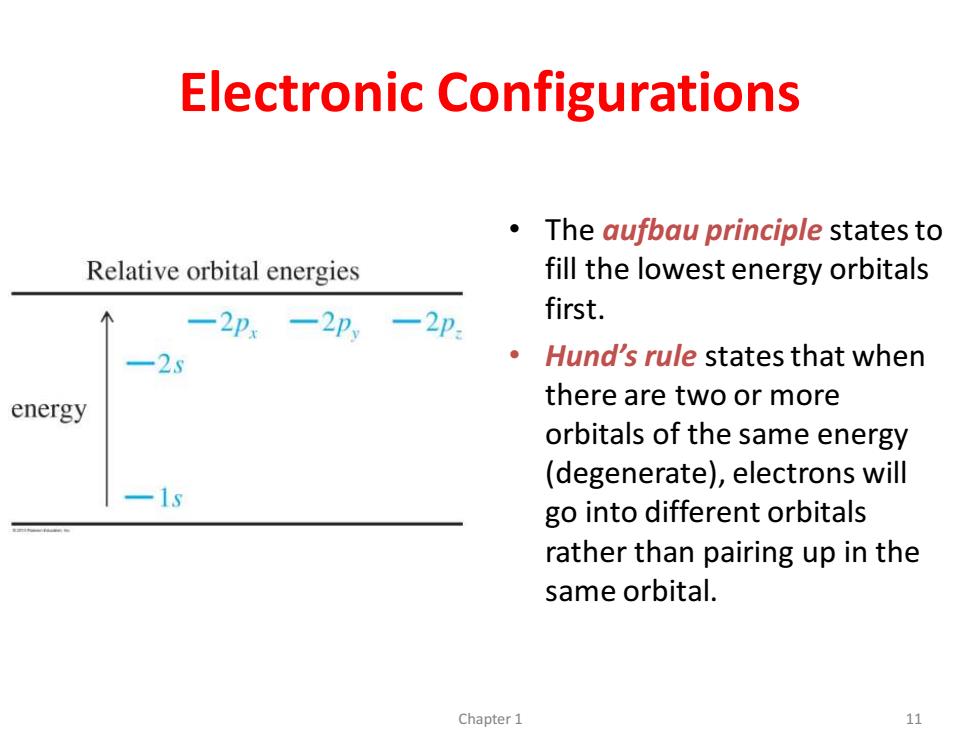
Electronic Configurations The aufbau principle states to Relative orbital energies fill the lowest energy orbitals 一2p,-2p一2p first. -25 Hund's rule states that when energy there are two or more orbitals of the same energy (degenerate),electrons will -1s go into different orbitals rather than pairing up in the same orbital. Chapter 1 11
Electronic Configurations • The aufbau principle states to fill the lowest energy orbitals first. • Hund’s rule states that when there are two or more orbitals of the same energy (degenerate), electrons will go into different orbitals rather than pairing up in the same orbital. Chapter 1 11
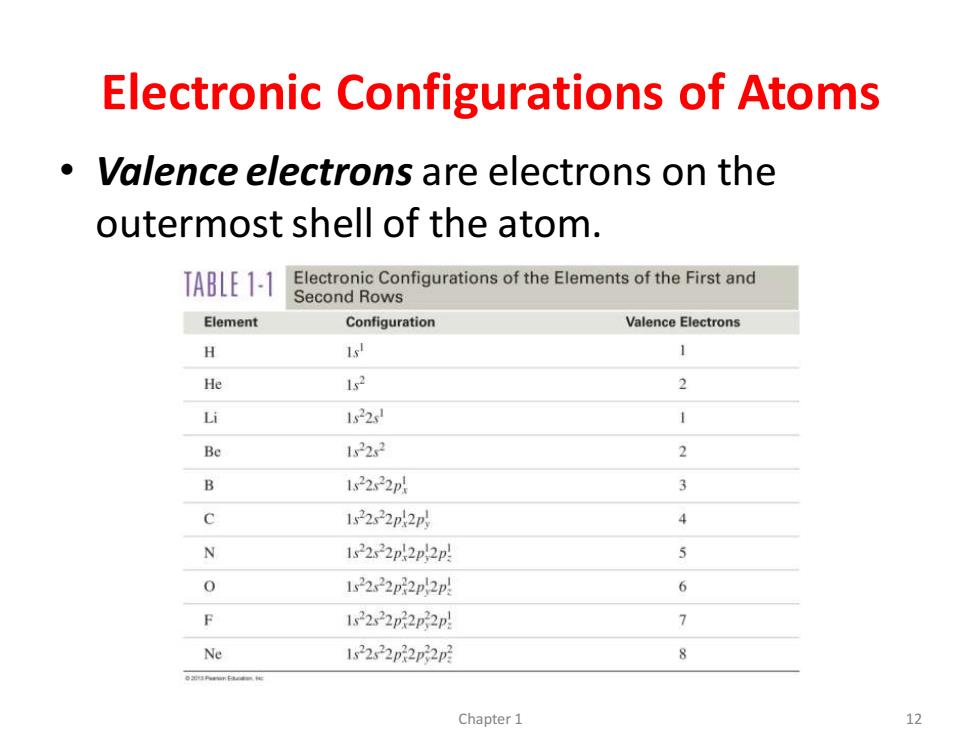
Electronic Configurations of Atoms Valence electrons are electrons on the outermost shell of the atom. TABLE 1-1 Electronic Configurations of the Elements of the First and Second Rows Element Configuration Valence Electrons H 16 1 He 152 2 Li 1522 1 Be 1522 2 B 12222p 3 0 12222p2p 4 N 1s2222p2p2p 5 0 1s2222p2p2p 6 12222p22p2p 1 Ne 1s222p2p2 8 Chapter 1 12
Electronic Configurations of Atoms • Valence electrons are electrons on the outermost shell of the atom. Chapter 1 12
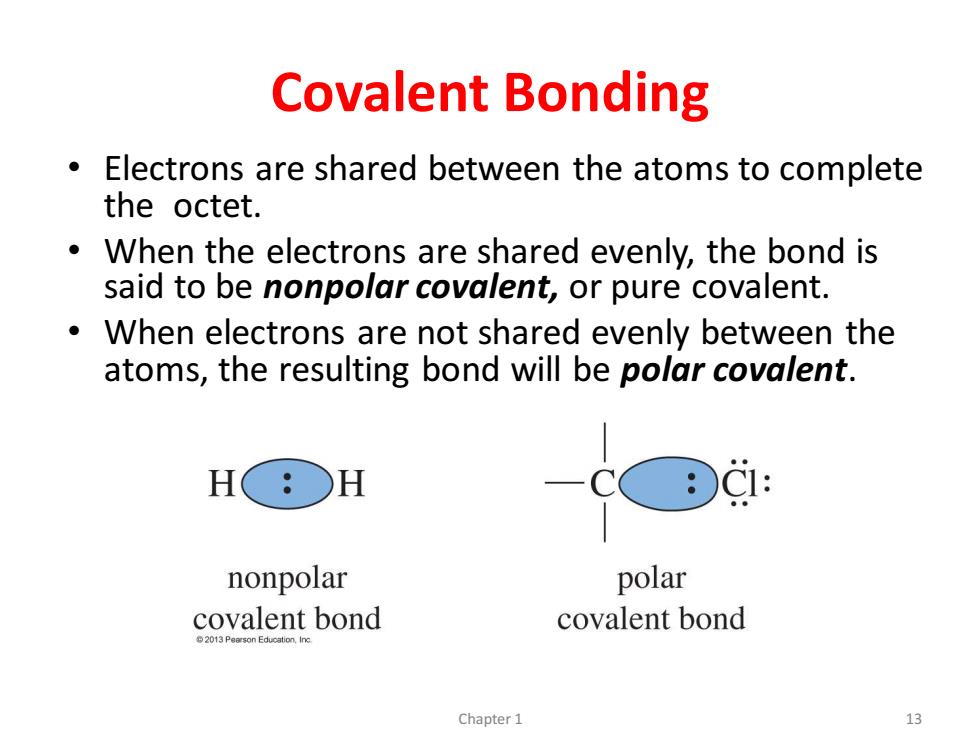
Covalent Bonding Electrons are shared between the atoms to complete the octet. ● When the electrons are shared evenly,the bond is said to be nonpolar covalent,or pure covalent. When electrons are not shared evenly between the atoms,the resulting bond will be polar covalent. HO: H nonpolar polar covalent bond covalent bond 2013 Pearson Education,Ing Chapter1 13
Covalent Bonding • Electrons are shared between the atoms to complete the octet. • When the electrons are shared evenly, the bond is said to be nonpolar covalent, or pure covalent. • When electrons are not shared evenly between the atoms, the resulting bond will be polar covalent. Chapter 1 13
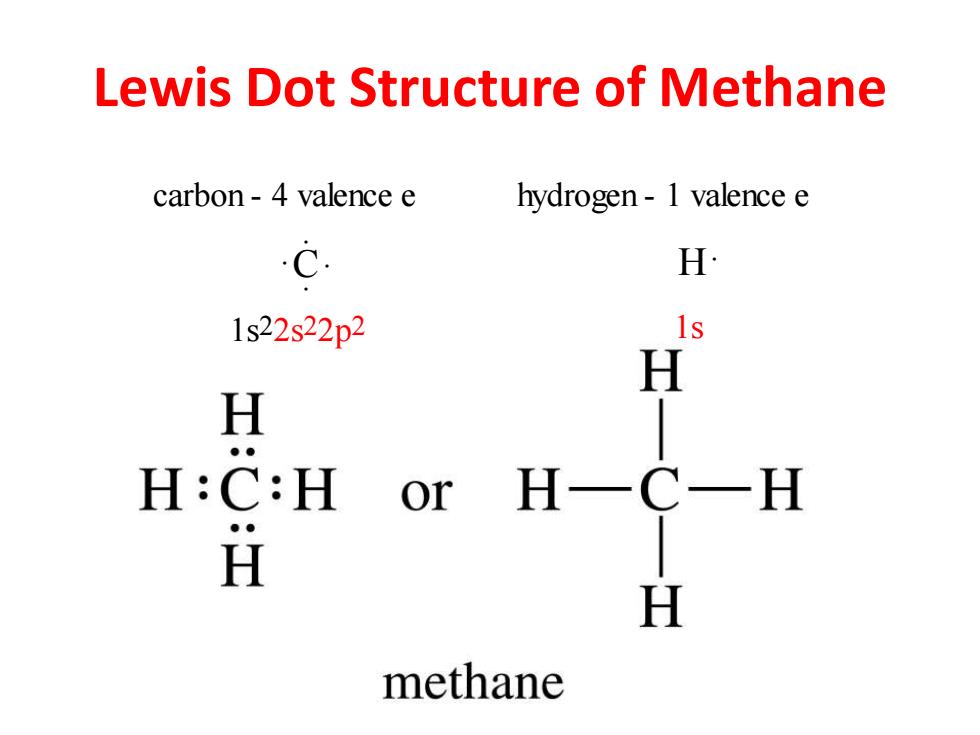
Lewis Dot Structure of Methane carbon-4 valence e hydrogen-1 valence e c H 1s22s22p2 1s H H H:C:H or HC H H H methane
Lewis Dot Structure of Methane C . . . . carbon - 4 valence e hydrogen - 1 valence e H . 1s2 2s2 2p2 1s
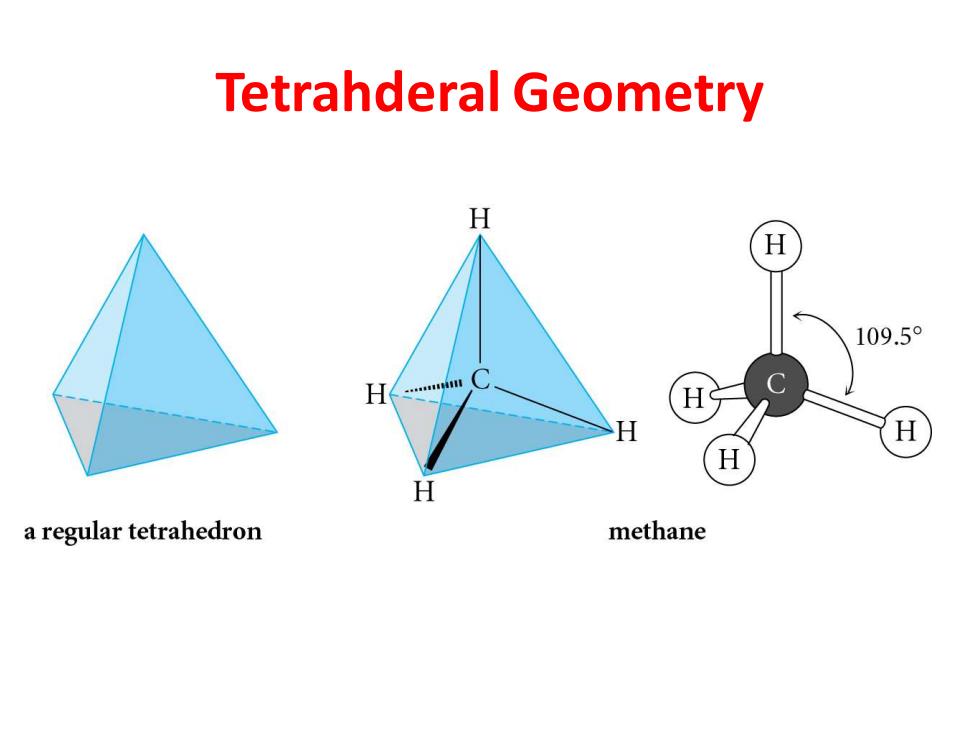
Tetrahderal Geometry H 109.5 H a regular tetrahedron methane
Tetrahderal Geometry