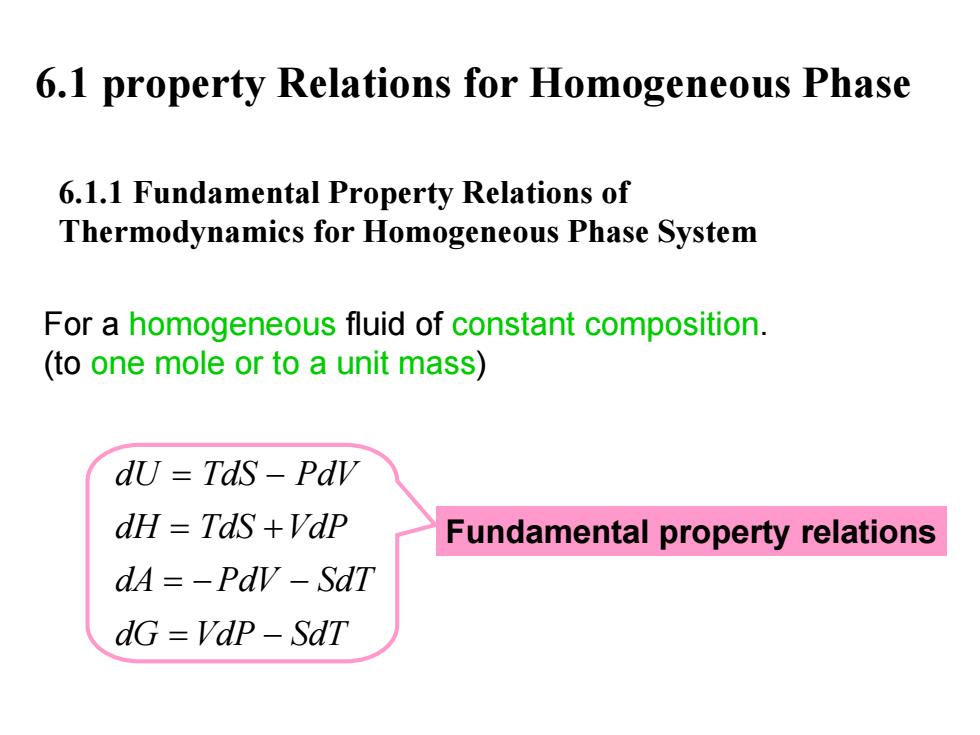
6.1 property Relations for Homogeneous Phase 6.1.1 Fundamental Property Relations of Thermodynamics for Homogeneous Phase System For a homogeneous fluid of constant composition. (to one mole or to a unit mass) dU Tds-Pdv dH TdS +VdP Fundamental property relations dA=-Pdv-SdT dG=VdP-SdT
6.1 property Relations for Homogeneous Phase 6.1.1 Fundamental Property Relations of Thermodynamics for Homogeneous Phase System For a homogeneous fluid of constant composition. (to one mole or to a unit mass ) dU TdS PdV dH TdS VdP dA PdV SdT dG VdP SdT = − = + =− − = − Fundamental property relations

Attention Constant-compositon Constant-mass 四个 小方程式,是我 用到的微分方程, 使用些方程时之安注意以下几点: 1.恒组分、恒质量体系,也就是封闭体系; 2.均相体系(单相1 Homogeneous phase 3.平衡态间的变化; Closed 4.常用于1摩尔时的性顶 system Change from one equalibrium state from to another cumoHhrium stoto
Attention ! Constant - compositon Homogeneous phase Closed system Constant - mass Change from one equalibrium state from to another equalibrium state
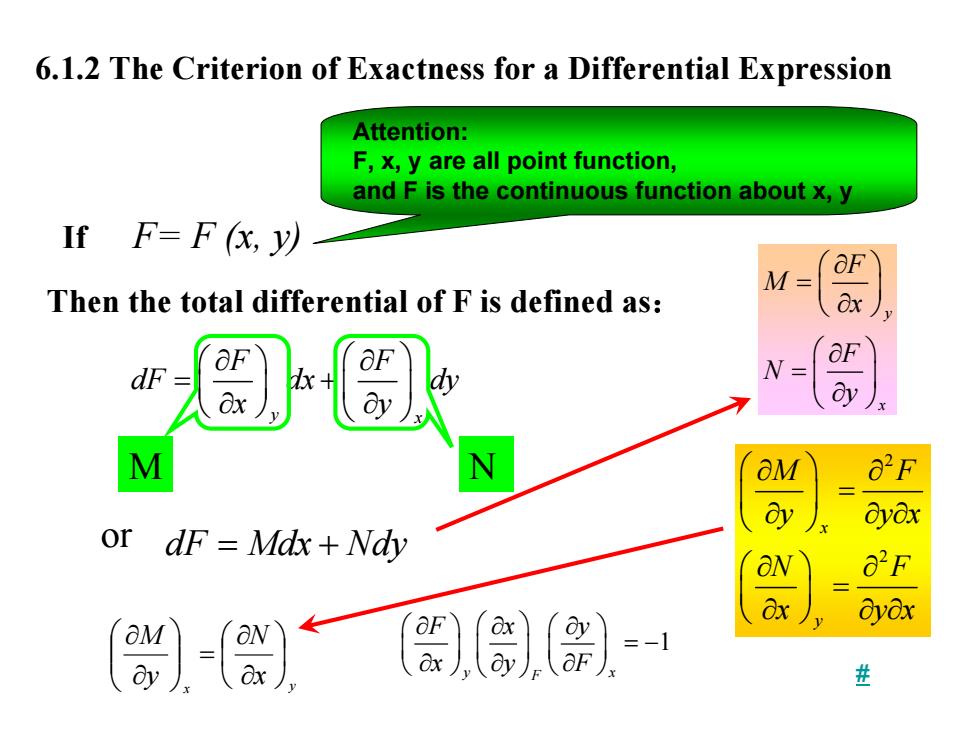
6.1.2 The Criterion of Exactness for a Differential Expression Attention: F,x,y are all point function, and F is the continuous function about x,y IfF=F(,y以 M= Then the total differential of F is defined as: &x OF dF ay M N OM Oyox or dF=Mdx+Ndy O"F ayox =-1
6.1.2 The Criterion of Exactness for a Differential Expression If F= F (x, y) y x F F dF dx dy x y ⎛ ⎞ ∂ ∂⎛ ⎞ = + ⎜ ⎟ ⎜ ⎟ ⎝ ⎠ ∂ ∂ ⎝ ⎠ dF Mdx Nd = + y Then the total differential of F is defined as: or 2 2 x y M F y y x N F x y x ⎛ ⎞ ∂ ∂ ⎜ ⎟ = ⎝ ⎠ ∂ ∂ ∂ ⎛ ⎞ ∂ ∂ ⎜ ⎟ = ⎝ ⎠ ∂ ∂ ∂ x y x N y M ⎟ ⎠ ⎞ ⎜ ⎝ ⎛ ∂ ∂ = ⎟ ⎟ ⎠ ⎞ ⎜ ⎜ ⎝ ⎛ ∂ ∂ y x F M x F N y ⎛ ⎞ ∂ = ⎜ ⎟ ⎝ ⎠ ∂ ⎛ ⎞ ∂ = ⎜ ⎟ ⎝ ⎠ ∂ M N 1 y x F Fx y xyF ⎛⎞ ⎛⎞ ∂∂∂ ⎛ ⎞ ⎜⎟ ⎜⎟ ⎜ ⎟ = − ⎝⎠ ⎝⎠ ∂∂∂ ⎝ ⎠ Attention: F, x, y are all point function, and F is the continuous function about x, y #
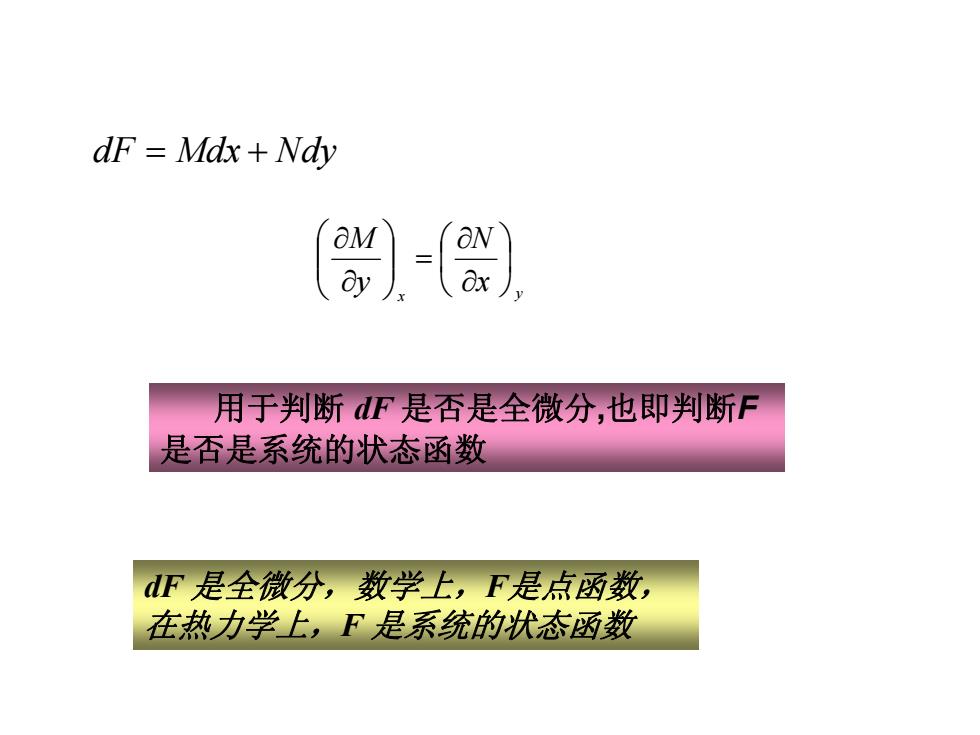
dF =Mdx Ndy aN 用于判断dF是否是全微分,也即判断F 是否是系统的状态函数 dF是全微分,数学上,F是点函数, 在热力学上,F是系统的状态函数
x y x N y M ⎟⎠⎞ ⎜⎝⎛ ∂∂ = ⎟⎟⎠⎞ ⎜⎜⎝⎛ ∂∂ dF Mdx Nd = + y 用于判断 dF 是否是全微分,也即判断F 是否是系统的状态函数 dF 是全微分,数学上,F是点函数, 在热力学上,F 是系统的状态函数
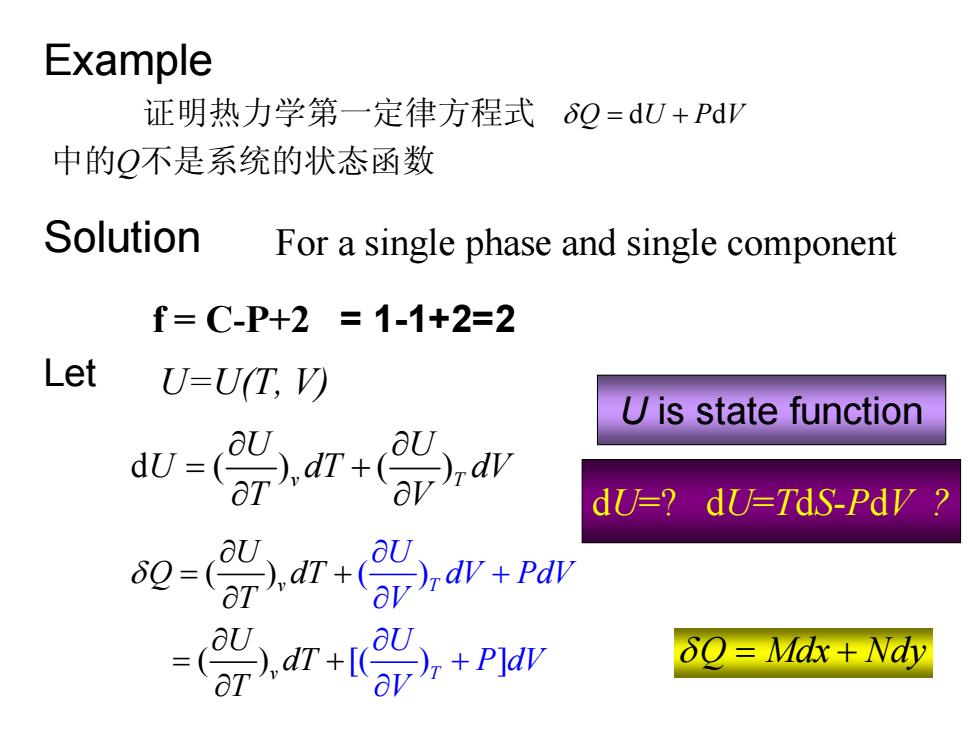
Example 证明热力学第一定律方程式6Q=dU+PdV 中的Q不是系统的状态函数 Solution For a single phase and single component f=C-P+2=1-1+2=2 Let U=U(T,V) U is state function -p. dU-?dU=TdS-Pdy d7+( U aU δQ=Mdx+Wdy
Example 证明热力学第一定律方程式 δQ U PV = d d + 中的Q不是系统的状态函数 Solution For a single phase and single component f = C-P+2 = 1-1+2=2 Let U=U(T, V) d () () v T U U U dT dV T V ∂ ∂ = + ∂ ∂ ( ) [( ) ( ) ] ) ( v T v T U dV PdV U Q dT T U dT V U d T P V V δ ∂ + ∂ ∂ + ∂ = + ∂ ∂ + ∂ = ∂ U is state function δQ Mdx Nd = + y dU=? dU=TdS-PdV ?