
上游充通大学 SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics I Lecture 36 Chapter 7 Entropy (Section7.10-7.11) Spring,2017 溺 Prof.,Dr.Yonghua HUANG MLAAMAA http://cc.sjtu.edu.cn/G2S/site/thermo.html
Engineering Thermodynamics I Lecture 36 Spring, 2017 Prof., Dr. Yonghua HUANG Chapter 7 Entropy (Section 7.10-7.11) http://cc.sjtu.edu.cn/G2S/site/thermo.html
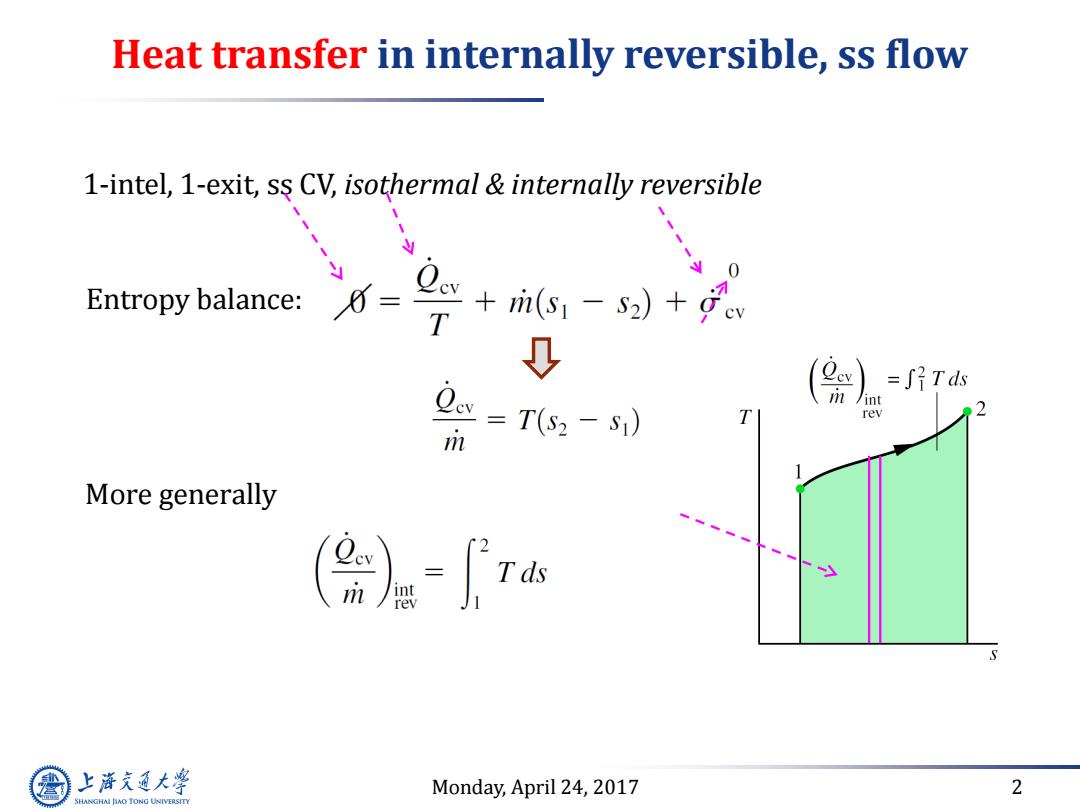
Heat transfer in internally reversible,ss flow 1-intel,1-exit,ss CV,isothermal internally reversible y Entropy balance: +i6,-s)+。 T 0 =∫?Tds w=T(s-s) /int rev m More generally ()= 上游究通大学 Monday,April 24,2017 2 SHANGHAI JIAO TONG UNIVERSITY
Monday, April 24, 2017 2 Heat transfer in internally reversible, ss flow 1-intel, 1-exit, ss CV, isothermal & internally reversible More generally Entropy balance:
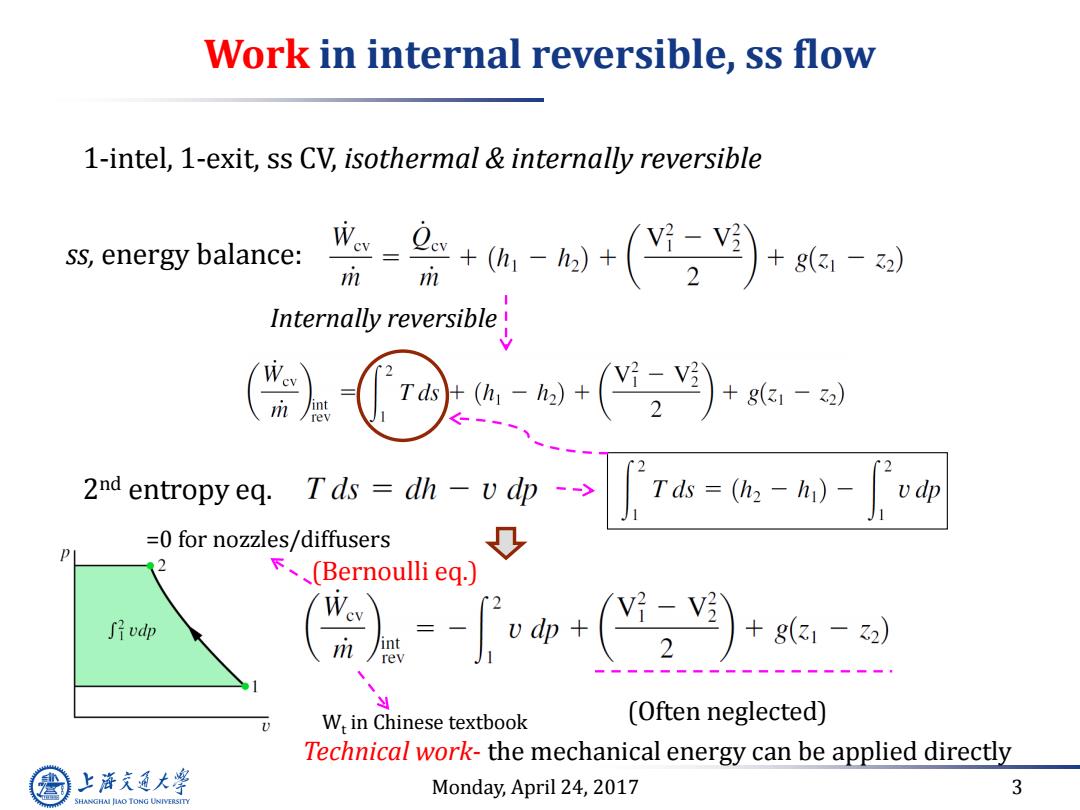
Work in internal reversible,ss flow 1-intel,1-exit,ss CV,isothermal internally reversible ss,energy balance: -品价+)+ m Internally reversible! m 2nd entropy eq.Tds =dh-v dp T=-)- vdp =0 for nozzles/diffusers 0 下、(Bernoulli eq, ?-V3 J斤vdp +g(31-2) W,in Chinese textbook (Often neglected) Technical work-the mechanical energy can be applied directly 上游充通大 Monday,April 24,2017 3 SHANGHAI JIAO TONG UNIVERSITY
Monday, April 24, 2017 3 Work in internal reversible, ss flow ss, energy balance: 1-intel, 1-exit, ss CV, isothermal & internally reversible Internally reversible 2 nd entropy eq. (Often neglected) Technical work- the mechanical energy can be applied directly Wt in Chinese textbook =0 for nozzles/diffusers (Bernoulli eq.)
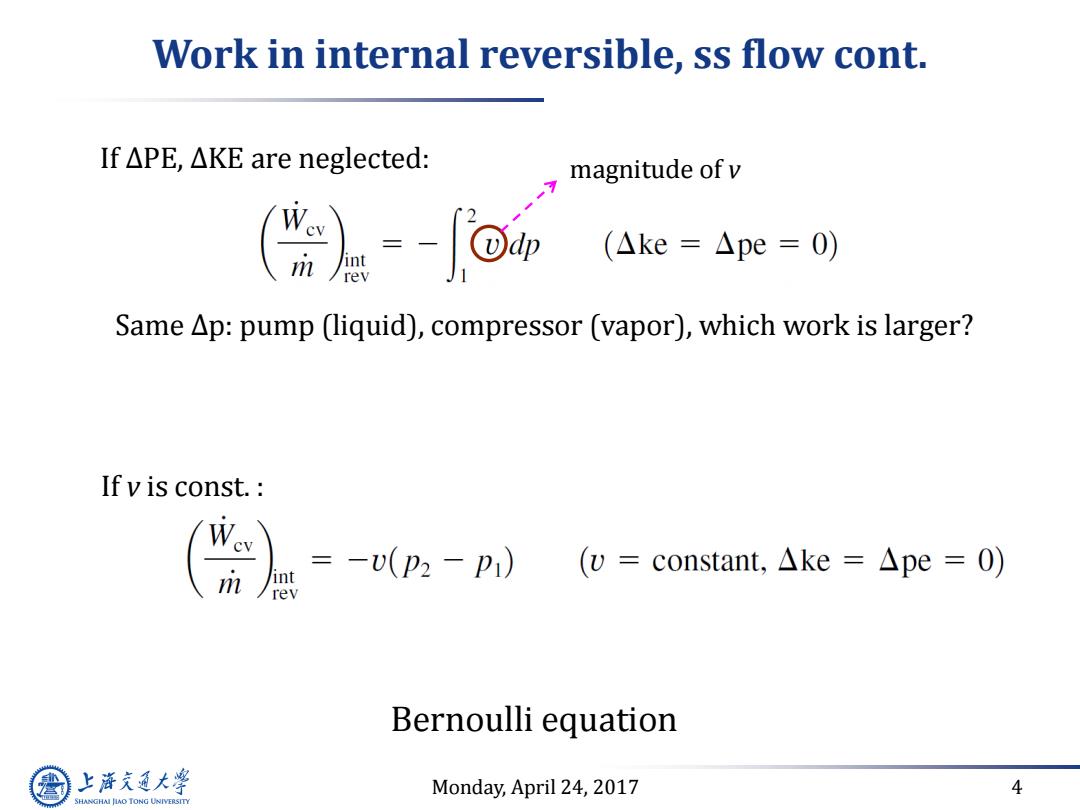
Work in internal reversible,ss flow cont. If△PE,△KE are neglected: magnitude of v (Ake=△pe=0) Same Ap:pump (liquid),compressor (vapor),which work is larger? If v is const. Wey m int =-U(p2-p1) (v=constant,.△ke=△pe=O) rev Bernoulli equation 上游究通大学 Monday,April 24,2017 4 SHANGHAI JLAO TONG UNIVERSITY
Monday, April 24, 2017 4 Work in internal reversible, ss flow cont. Bernoulli equation If ∆PE, ∆KE are neglected: If v is const. : Same ∆p: pump (liquid), compressor (vapor), which work is larger? magnitude of v
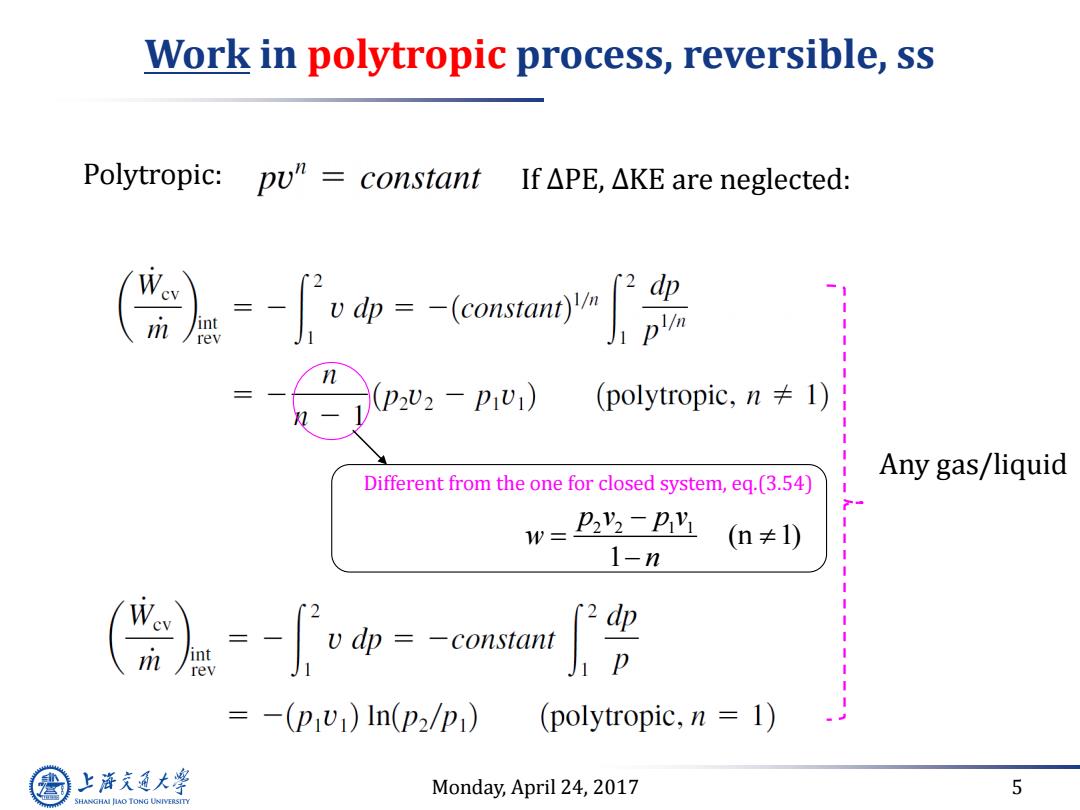
Work in polytropic process,reversible,ss Polytropic:pu"constant If△PE,△KE are neglected: -=-omm -1 m rev p2U2-p1U1) (polytropic,n≠l) Any gas/liquid Different from the one for closed system,eq.(3.54) w=22v-pv (n≠1) 1-n =-(p1w)ln(p2/p1) (polytropic,n 1) 上降文通大学 Monday,April 24,2017 5 SHANGHAI JIAO TONG UNIVERSITY
Monday, April 24, 2017 5 Work in polytropic process, reversible, ss Polytropic: If ∆PE, ∆KE are neglected: Any gas/liquid Different from the one for closed system, eq.(3.54) 2 2 1 1 (n 1) 1 p v p v w n