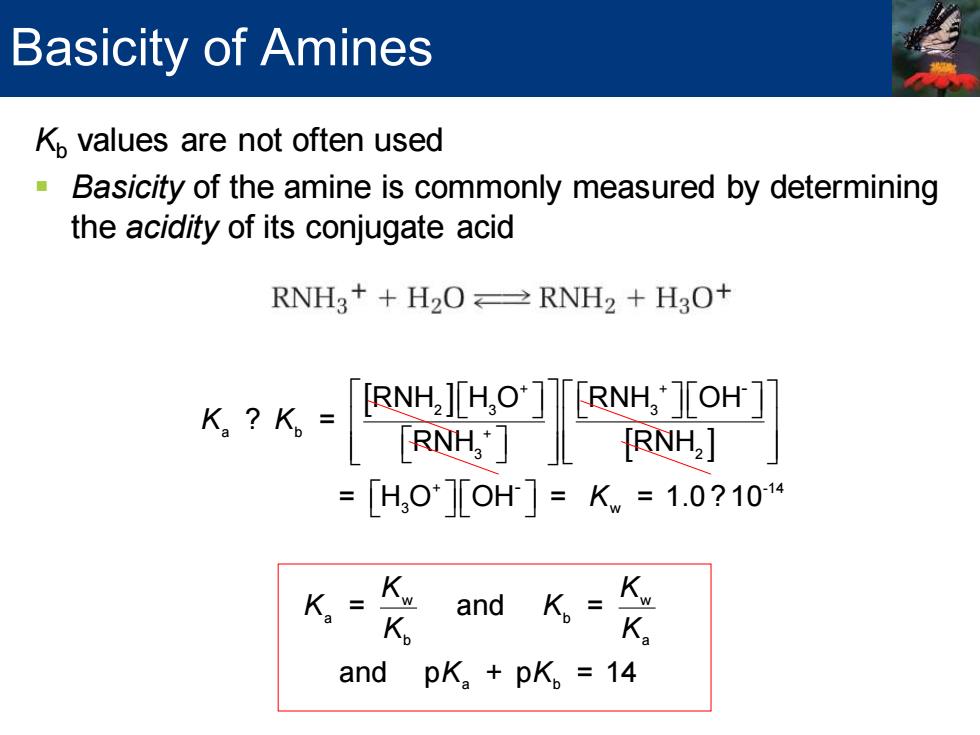
Basicity of Amines Kp values are not often used Basicity of the amine is commonly measured by determining the acidity of its conjugate acid RNHg++H2O≌RNH2+H3O+ 6k-90鬥 =[H,0][OH]=Kw=1.0?104 K= K and K.=K. K and pK.+pK。=14
Kb values are not often used ▪ Basicity of the amine is commonly measured by determining the acidity of its conjugate acid + + 3 2 2 3 + + 2 3 3 a b + 3 2 + -14 3 w w a b - - RNH + H O RNH + H O RNH H O RNH OH ? = RNH RNH = H O OH = = 1.0 ? 10 = K K K K K K w b a a b and = and p + p = 14 K K K K K Basicity of Amines
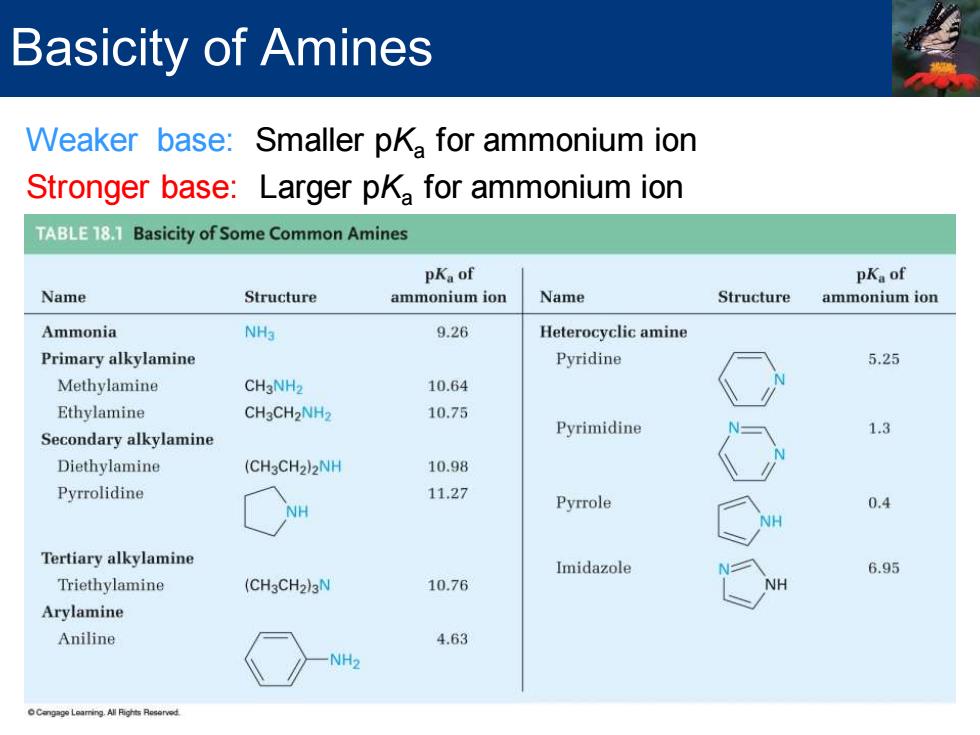
Basicity of Amines Weaker base:Smaller pKa for ammonium ion Stronger base:Larger pKa for ammonium ion ABLE181 Basicity of Some Common Amines pKa of pKa of Name Structure ammonium ion Name Structure ammonium ion Ammonia NH3 9.26 Heterocyclic amine Primary alkylamine Pyridine 5.25 Methylamine CH3NH2 10.64 Ethylamine CHgCH2NH2 10.75 Secondary alkylamine Pyrimidine 1.3 Diethylamine (CH3CH2)2NH 10.98 Pyrrolidine 1127 H Pyrrole 0.4 Tertiary alkylamine Imidazole 6.95 Triethylamine (CH3CH2)3N 10.76 Arylamine Aniline 4.63 NH2
Weaker base: Smaller pKa for ammonium ion Stronger base: Larger pKa for ammonium ion Basicity of Amines
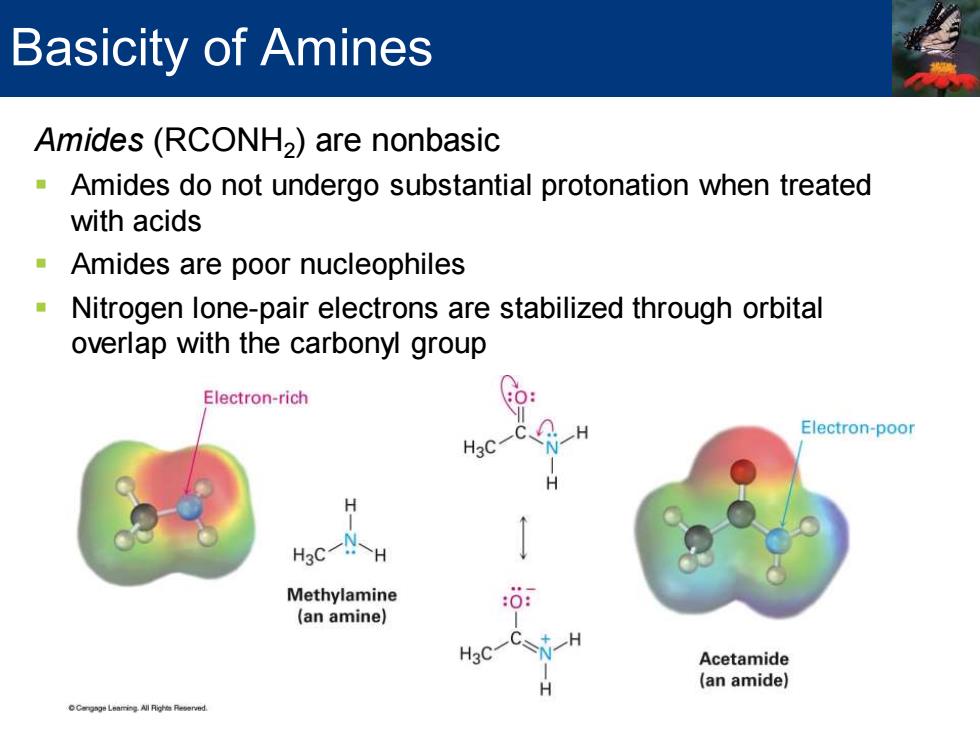
Basicity of Amines Amides(RCONH2)are nonbasic Amides do not undergo substantial protonation when treated with acids Amides are poor nucleophiles Nitrogen lone-pair electrons are stabilized through orbital overlap with the carbonyl group Electron-rich Electron-poor H30 Hc一H Methylamine (an amine) Acetamide (an amide) geLemng All Righs Reerved
Amides (RCONH2 ) are nonbasic ▪ Amides do not undergo substantial protonation when treated with acids ▪ Amides are poor nucleophiles ▪ Nitrogen lone-pair electrons are stabilized through orbital overlap with the carbonyl group Basicity of Amines
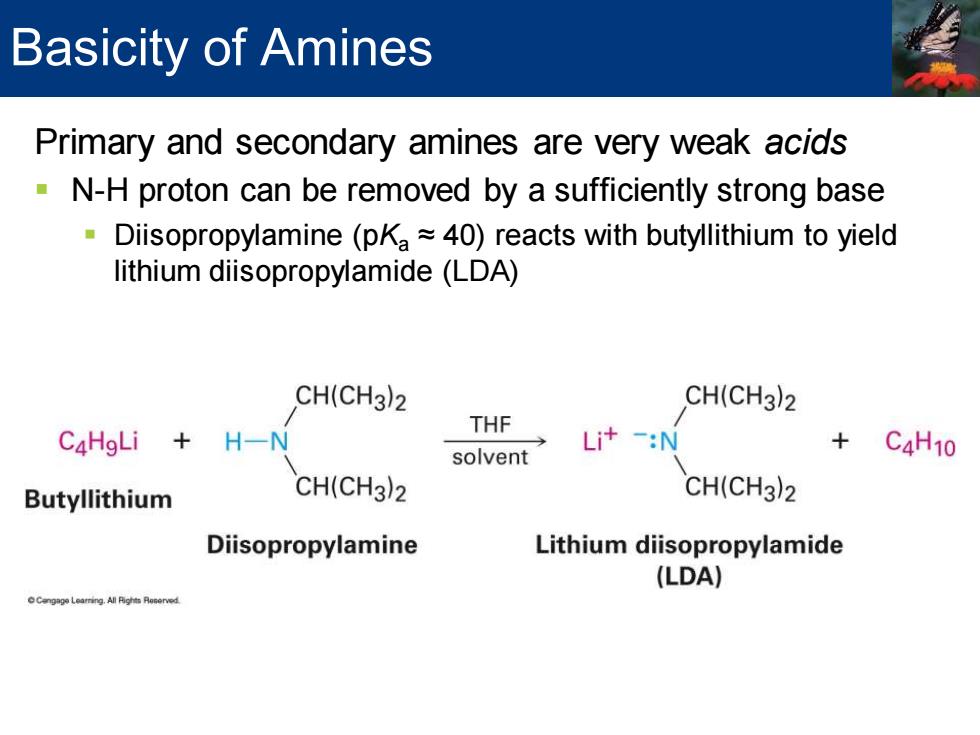
Basicity of Amines Primary and secondary amines are very weak acids N-H proton can be removed by a sufficiently strong base Diisopropylamine (pKa~40)reacts with butyllithium to yield lithium diisopropylamide(LDA) CH(CH3)2 CH(CH3)2 THF C4HgLi H-N Li+-:N solvent C4H10 Butyllithium CH(CH3)2 CH(CH3)2 Diisopropylamine Lithium diisopropylamide (LDA)
Primary and secondary amines are very weak acids ▪ N-H proton can be removed by a sufficiently strong base ▪ Diisopropylamine (pKa ≈ 40) reacts with butyllithium to yield lithium diisopropylamide (LDA) Basicity of Amines
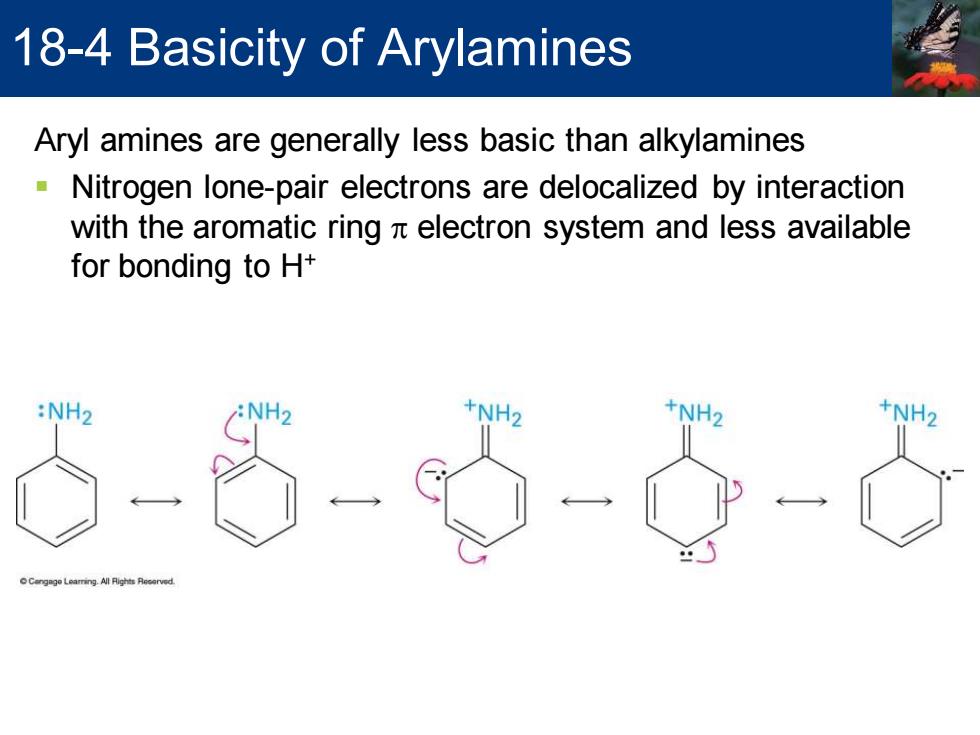
18-4 Basicity of Arylamines Aryl amines are generally less basic than alkylamines Nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring x electron system and less available for bonding to H+ :NH2 +NH2 +NH2
Aryl amines are generally less basic than alkylamines ▪ Nitrogen lone-pair electrons are delocalized by interaction with the aromatic ring p electron system and less available for bonding to H+ 18-4 Basicity of Arylamines