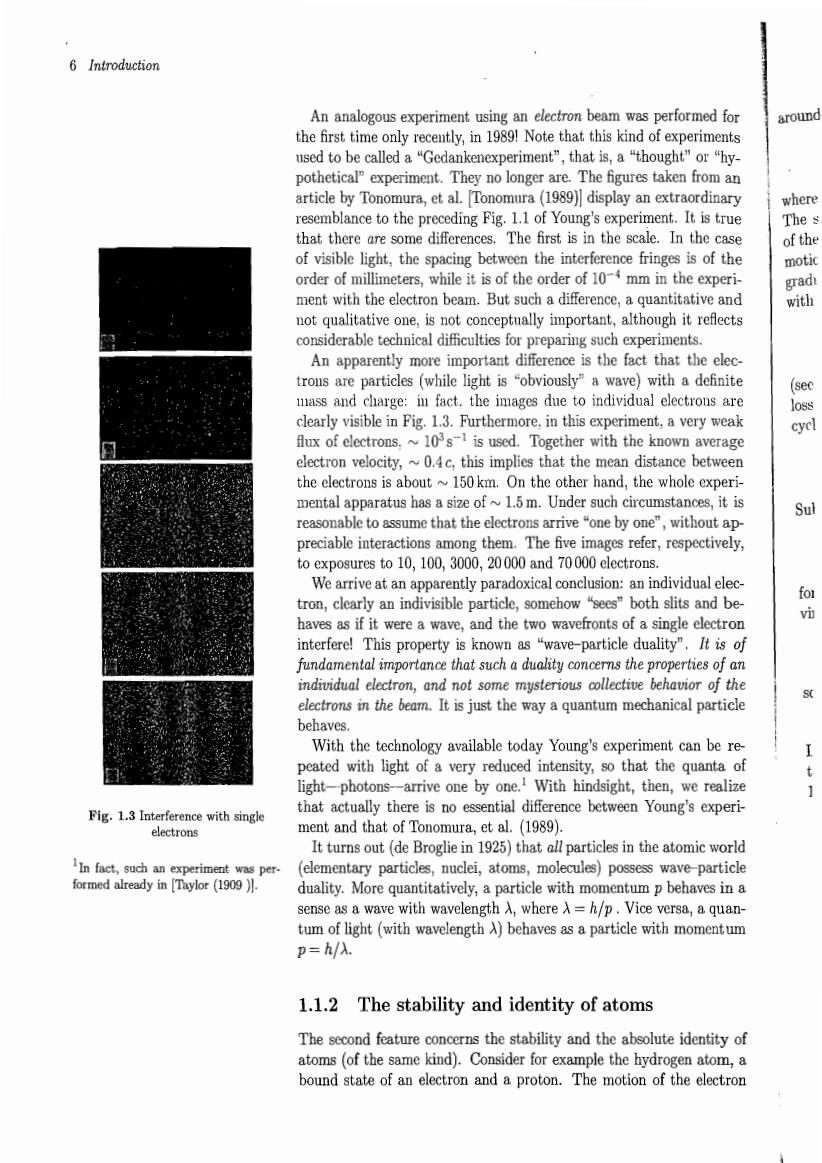
6 Introduction An analogous experiment using an electron beam was performed for around the first time only recently,in 1989!Note that this kind of experiments used to be called a"Gedankenexperiment",that is,a "thought"or"hy- pothetical"experiment They no longer are.The figures taken from an article by Ton mura et al.Tonomura (1989)display an extraordina t.It is tru that there are The first is in th scale.In the orde s is of the m0 tive one,is n t conceptually important An apparently more important difference is the fact that the elec trous are particles (while light is "obviously"a wave)with a definite mass and charge:in fact.the images due to individual electrons are clearly visible in Fig.1.3.Furthermore,in this experiment,a very weak flux of electrons.~10s1 is used.Together with the known average electron velocity,~0.4c,this implies that the mean distance between the electrons is about~150km.On the other hand,the whole experi- mental apparatus has a size of1.5m.Under such circumstances,it is reasonable to assume that the electrons arrive"one by one",without ap- to exposures to 10,100.3000.20000 and 70000 electrons. arently paradoxica:individu ticle.somehow "sees"both slits and be as if it were aw and the tw paefoatsofaSmged property is kn aveparticleduali It is rties osne地 an mystertous col r时th It is just the way aquantu mechanical particl e With the technology available today Young's experiment can be re peated with light of a very reduced intensity,so that the quanta of light-photons-arrive one by one.With hindsight,then,we realize Fig.with singe that actually there is no essential difference between Young's experi- ment and that of Tonomura,et al.(1989). It turns out(de Broglie in 1925)that all particles in the atomic world In fact,such an ex (elementary particles,nuclei,atoms,molecules)possess wave-particle duality.More quantitatively,a particle with momentum p behaves in a sense as a wave with wavelength a.where a=h/p.vice versa.a quan- tum of light(with wavelength A)behaves as a particle with momentum m=h/ 1.1.2 The stability and identity of atoms The second feature concerns the stability and the absolute identity of e kind).Consider for f an electron and a proton The motion

1.1 The quantum behavior of the electron 7 around the nucleus is described,in classical mechanics,by the equation m并= +mr的 r26=const.. (1.1) where for simplicity we have assumed a circular motion with radius r. The static Coulomb force is,however,just one of the manifestations radually.An orbit with constantr would not be stable.For an electron 修 (1.2) (see [Landau and Lifshitz (1976 b)).Let us assume also that the energy loss is so sinall that the orbit can be assumed to be circular for many cycles.From eqn (1.1)one finds that = mr2 Substitution of this into(1.)yields 9=9-3 for the energy loss per unit time interval.But for circular motion,the virial theorem tells us that e2 fan $ so that 4e4 --3md Lettbe the time needed for the complete collapse,(t)=0,assuming that at the beginning the system had the radius of a typical atom,a 10-8 cm.Integrating the equation,one gets happens in Nature. reason for whicheqn(1.2)does not apply to the atomic world-after all. Maxwell's theory was discovered in the macroscopic world-and ignoring the aforementioned difficulty,there is another,very serious,problem with the "planetary"model of atoms.It lies in the fact that each atom will have a different,quite random radius,depending on how it has been y of formed (i.e.,depending on the initial conditions). As we shall see below,all periodic motions are actually "quantized" only a certain discrete set of orbits is allowed.As a consequence,all
8 Introduction the atoms of the same kind,in the normal state (i.e.,with the lowest ands' energy av e is a qua ntum orb e8. the elect cannot further Qua energy,having no lower states to go the reason why eqn (1.1)cannot have a solution or characteristic,radius,independent of the mitia conditions.With ony dimensional parameters m and e at our disposal, 1.2 with dimensions Befor m=Igl:e=lg!P2cm32s-1] devek it is not possible to form any quantity with the dimension of length.In heat 2rent临Pan unde led t stor gcm2/s=erg·s, which will enter dynamical equations.Its numerical value is given by 12 h=6.62606896(3)×10-27(ergs. h2 B=42m2 ten oB咖 r rB≌5×10-9cm (1.3) g which is quite reasonable as the dimension of an atom. The absolutely identical intrinsic properties of elementary particles, atoms,or molecules,of the same type,are at the basis of the regularity and stability of macroscopic world (crystals,stars and planets,biological systems,etc.)Without such regularity,biological phenomena which require an exceedingly high precision in the structures at the microscopic N level,would not be possible.The simplicity in the microscopic world is in clear contrast,and at the same time in harmony,with the infinite variety of the macroscopic world we live in. t 1.1.3 Tunnel effects The third example is related to the familiar phenomenon of electrical conduction. In a simplified model,the electrons move in a periodic potential like Fig.1.4.which is a one-dim ion of a ig.1.4A periodic potentia netallic c tal lattice A classical particle withr less than the from one tial w mal field ngh to rcome th fa像诚s任5做gse2 were electron,aqu tum mechanical part icle/wave
1.2 The birth of quantum mechanics 9 bands"of allowed values),allowing electrical conduction.This is an 6 ofhtcnd is clely relted to the Quantum mechanics describes these and many other phenomena of the microscopic nl 1.2 The birth of quantum mechanics Before presenting quntum mechanics itsef,however,let us discuss the developments which took place in the years 1900-1925.The efforts in. volved in trying to solve the difficulties of the classical theory of specific heat and of the so-called black-body radiation,as well as in trying to understand the mysteries of atomic spectral lines,which all eventually led to the formulation of quantum mechanics,make for an extraordinary story of scientific discovery. 1.2.1 From the theory of specific heat to Planck's formula Let us consider a macroscopic system described by the- order of the Avoga mber of degr ffreedom is large ro A≥6×1023 with a heat bath, B0 of the systen Ac mann's theory,the prob found +s脸血a+n内+ in the mi 3) (1.4) where N is the normalization factor.=1.380658 x 10-23 J.K-1 is the Boltzmann conversion constant. The "equipartition law follows from Boltzmann's law immediately. Namely,for a system described by a Hamiltonian of the form H=∑au+ag (1.5) the mean values of each term of the Hamiltonian turn out to be equal (anp)=(2)=T,(indep.of n), ywe cap ay thath心dom eth IkT of the he classical t simple he n law. e gases, =0,while for diatomic gases suc as H,etc.,the expression for the energy is B=∑所马=民+场+是+3+/血 2m 2

10 Introduction where the last two terms represent the rotational degrees of freedom(we the sys neglect here the vibrational modes between the two atoms).The total energy for a mole is then L,the U=KTNA=RT:and U=KTNA=RT, k respectively,for the monoatomic and diatomic gases,NA is the Avogadro see Su number,and (1.5). the an R=NAk8.31441 x 107 ergmol-K-1 =1.987 calmol-K-1 is the gas constant.The specific heat is then given in the two cases by and t R2.98.monoatomic gases, 7 diatounic gaoes In ot in good rdiaopeganrelo t below the pre tomic gas ases and ap 150K Analogous results were found temperature,and tends to zero as the temperature approaches zero In all cases,then,it seems that at any given temperature not all of the degrees of freedom get the shareT expected from the equipartition law.As the temperature lowers,more degrees of freedom get frozen and eventually all of the degrees of freedom die out. In the last decade of the 19th century,the failure of classical physics became more widely acknowledged owing to the problem of black-body radiation Consider an empty cavity kept at a fixed temperature t.its interior fre is filled with electromagnetic radiation,in thermal equilibrium with the heat bath(the wall of the cavity).Now what is the color of the light inside?What is the and relative intensity of light the 8 How much beat is eded to ir of the interior of degree("th cific heat of the uum"? b)) H=e+的a By the appropriate bound ary condition for perfectly refiecting boundary for simplicity of calcula tion,we find that the above Hamiltonian can be cast in the form H=∑(后+ka)+∑(后%+)