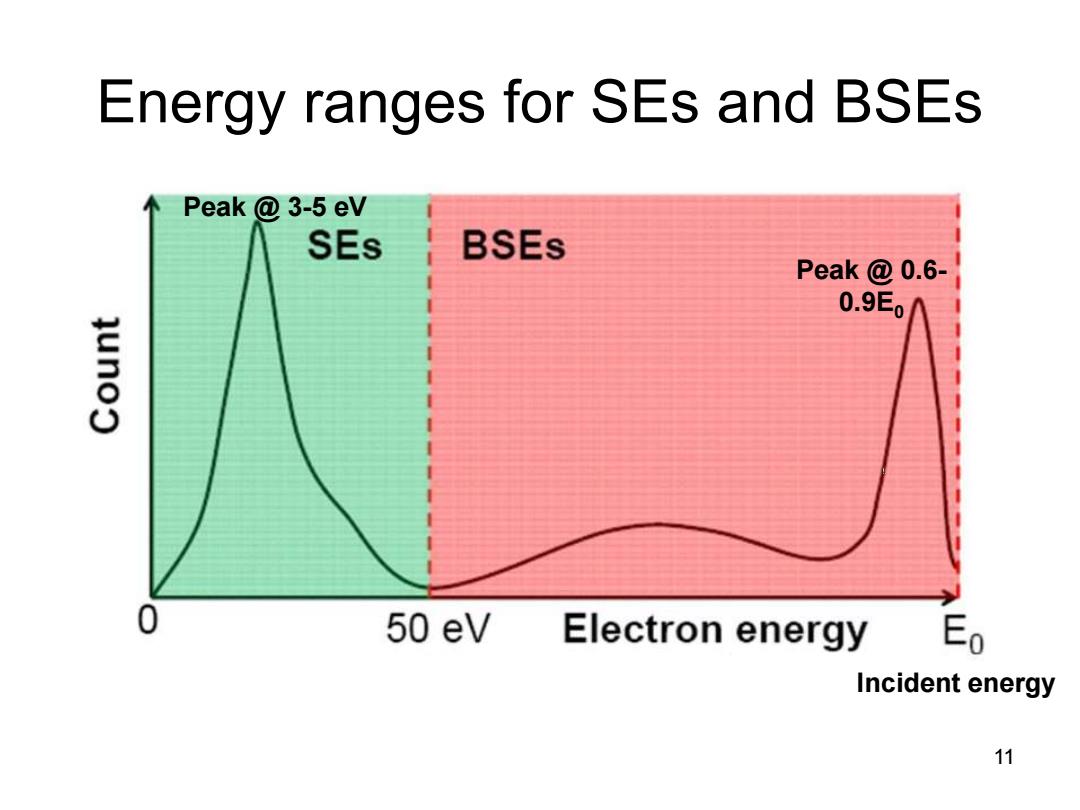
Energy ranges for SEs and BSEs Peak 3-5 eV SEs BSEs Peak @0.6- 0.9E 0 50 eV Electron energy Eo Incident energy 11
Peak @ 3-5 eV Peak @ 0.6- 0.9E0 Incident energy Energy ranges for SEs and BSEs 11

Characteristic X-rays An electron is ejected by the incident beam and replaced by an electron from an upper layer,emitting a photon of energy equal to the difference of energy between the two layers. X-Ray KB LB Ka L23 L K Shell ● Nucleus 12
Characteristic X-rays • An electron is ejected by the incident beam and replaced by an electron from an upper layer, emitting a photon of energy equal to the difference of energy between the two layers. 12
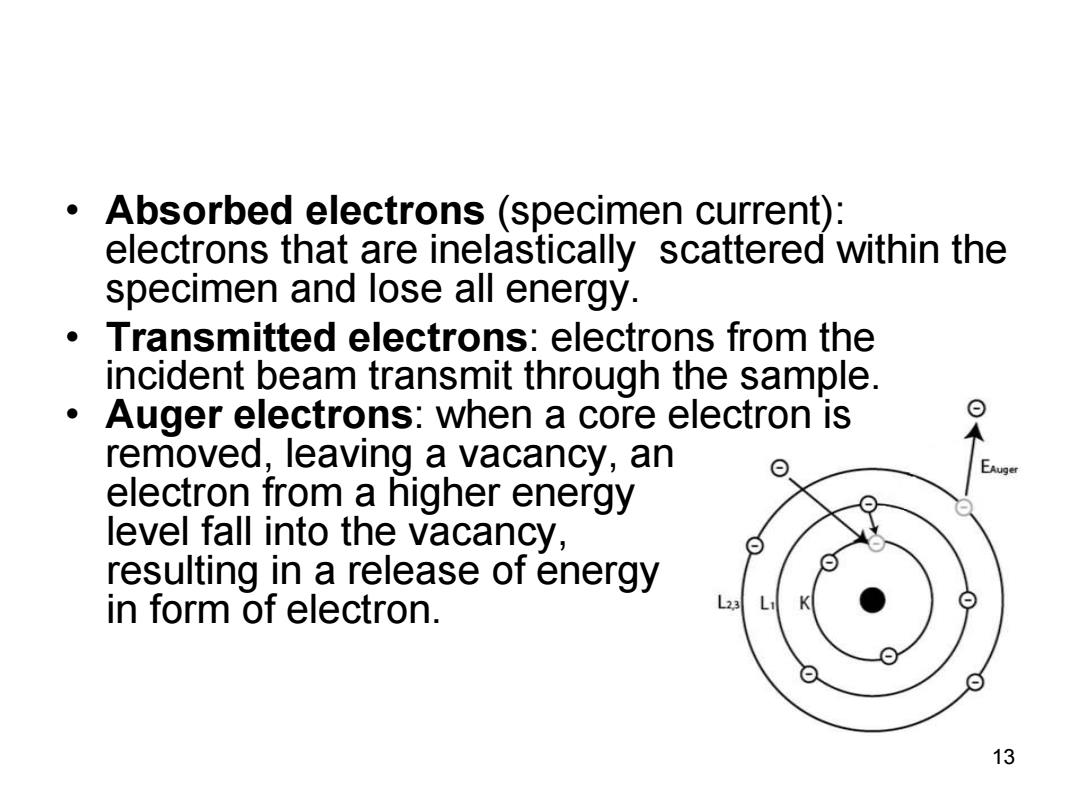
Absorbed electrons (specimen current): electrons that are inelastically scattered within the specimen and lose all energy. Transmitted electrons:electrons from the incident beam transmit through the sample. Auger electrons:when a core electron is removed,leaving a vacancy,an electron from a higher energy level fall into the vacancy, resulting in a release of energy in form of electron. L23 L ⊙ 13
• Absorbed electrons (specimen current): electrons that are inelastically scattered within the specimen and lose all energy. • Transmitted electrons: electrons from the incident beam transmit through the sample. • Auger electrons: when a core electron is removed, leaving a vacancy, an electron from a higher energy level fall into the vacancy, resulting in a release of energy in form of electron. 13

Sample surface 75V 0.2μm 15kw1.0μm 20kw2.4μm 30 kV 3.1μm Penetration depth in Fe Relationship of beam energy and reaction volume 14
Sample surface Relationship of beam energy and reaction volume Penetration depth in Fe 3.1 μm 1.0 μm 2.4 μm 0.2 μm 14

The size of the interaction volume decreases as the average atomic number of the material increases Note also the shape change Fe(Z=26) Ag(Z=47) Monte Carlo simulations for a 20 keV incident beam c(Z=6) Relationship of beam energy and Z 15
Relationship of beam energy and Z 15