
上海通大学 Shanghai Jiao Tong University 但.… ©但硅氧化形成的S0,在地球温度范围内多数以固体状态存在,而C02可以以气 体的形式轻易释放。 ©硅化合物的硬度太高,使得支撑生命所需的链间反应难以实现。而碳形成碳水 化合物(作为能源),酶(作为催化剂),以及手性特征满足在生物体内各种化学反 应及调控的需要 ©缺乏硅态生命甚至硅态前生命化学过程已经被天体学证据所证明。在陨石、彗 星、巨星大气、星际物质和冷却星球表面,发现有二氧化硅,但没有找到SH4 或硅酮等前生命化学过程的物质
Shanghai Jiao Tong University 但硅氧化形成的SiO2 在地球温度范围内多数以固体状态存在,而CO2 可以以气 体的形式轻易释放。 硅化合物的硬度太高,使得支撑生命所需的链间反应难以实现。而碳形成碳水 化合物 (作为能源), 酶 (作为催化剂), 以及手性特征满足在生物体内各种化学反 应及调控的需要 缺乏硅态生命甚至硅态前生命化学过程已经被天体学证据所证明。在陨石、彗 星、巨星大气、星际物质和冷却星球表面,发现有二氧化硅,但没有找到SiH4 或硅酮 等前生命化学过程的物质。 但…

上游充通大¥ 硅和碳的其它差别 Shanghai Jiao Tong University ·Si极少形成双键或三键,但他们是碳生命形式的主要特征(Amino acids, fatty acids and nucleotides frequently have double bonds between C&O). ● 没有发现硅态芳香族化合物aromatic compounds ·全氧化态硅SO2是一种惰性物质. ·在氧存在的情况下SiH4解离形成SiO2 水不能溶解硅化合物 benzene Tap view Side view
Shanghai Jiao Tong University • Si 极少形成双键或三键, 但他们是碳生命形式的主要特征 (Amino acids, fatty acids and nucleotides frequently have double bonds between C & O). • 没有发现硅态芳香族化合物 aromatic compounds • 全氧化态硅 SiO2 是一种惰性物质. • 在氧存在的情况下SiH4 解离形成 SiO2 . • 水不能溶解硅化合物 benzene 硅和碳的其它差别

上游充通大¥ 为什么需要溶剂 Shanghai Jiao Tong University 生命需要溶剂的原因: 1.液体环境有利于一些化学键的稳定性一保持生物大分子的结构。 2.它促进了其他一些化学键的解离有利于化学交换与能量转移。能使很多物质溶 解而不溶解生物大分子,从而提供了边界、表面、界面及立体化学的稳定性。 3.液体的密度能够使反应物保持一定的浓度并控制它们的扩散。 ④.液体能够为生化反应提供一个维持温度/压力上下限的介质有利于代谢途径的优 化 5.液体能够缓冲环境变化所带来的影响
Shanghai Jiao Tong University 生命需要溶剂的原因: 1. 液体环境有利于一些化学键的稳定性一保持生物大分子的结构。 2. 它促进了其他一些化学键的解离有利于化学交换与能量转移。能使很多物质溶 解而不溶解生物大分子,从而提供了边界、表面、界面及立体化学的稳定性。 3. 液体的密度能够使反应物保持一定的浓度并控制它们的扩散。 4. 液体能够为生化反应提供一个维持温度/压力上下限的介质有利于代谢途径的优 化 5. 液体能够缓冲环境变化所带来的影响. 为什么需要溶剂
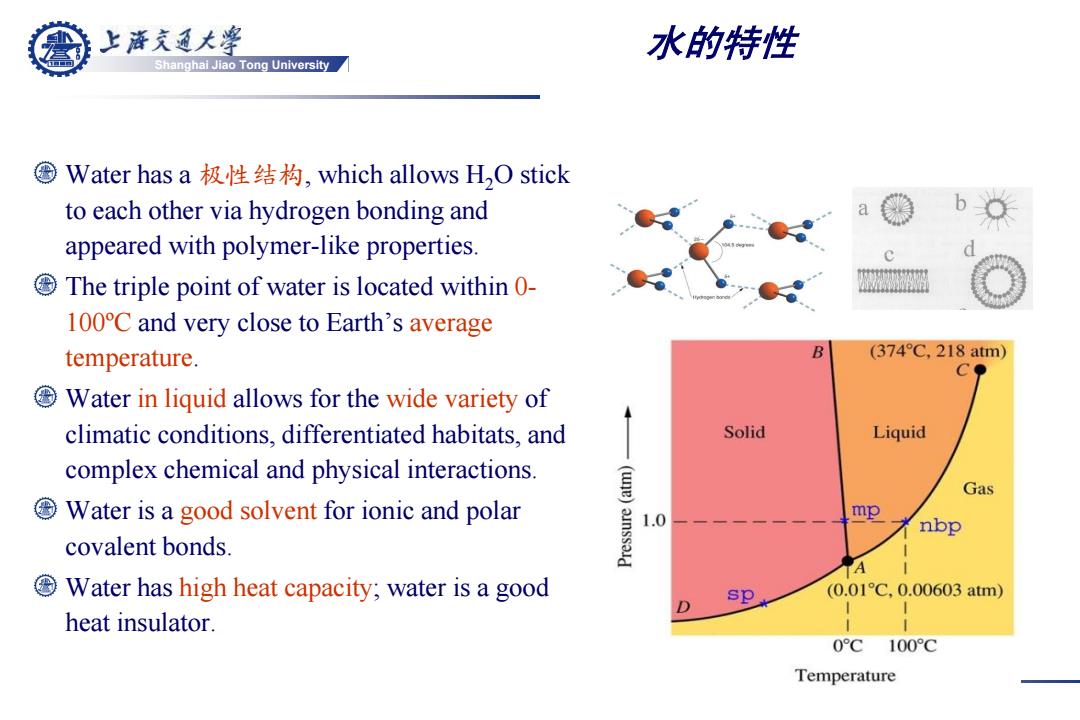
上游充通大¥ 水的特性 Shanghai Jiao Tong University ©Water has a极性结构,which allows H2O stick to each other via hydrogen bonding and appeared with polymer-like properties. The triple point of water is located within 0- 100C and very close to Earth's average temperature. (374°C,218atm) Water in liquid allows for the wide variety of climatic conditions,differentiated habitats,and Solid Liquid complex chemical and physical interactions. 息 Gas Water is a good solvent for ionic and polar 1.0 mp nbp covalent bonds. A Water has high heat capacity;water is a good sp (0.01C,0.00603atm) heat insulator. 0C 100°C Temperature
Shanghai Jiao Tong University Water has a 极性结构, which allows H2O stick to each other via hydrogen bonding and appeared with polymer-like properties. The triple point of water is located within 0- 100ºC and very close to Earth’s average temperature. Water in liquid allows for the wide variety of climatic conditions, differentiated habitats, and complex chemical and physical interactions. Water is a good solvent for ionic and polar covalent bonds. Water has high heat capacity; water is a good heat insulator. 水的特性

水作为类地球环境中生命所需最好的溶剂 Ice is lighter than liquid water.The ice cover acts as insulator that prevents the complete freezing of a body of water from the bottom to the top. High surface tension.Organic compounds may be concentrated in small water droplets which enhanced 10 20 30 。zone(DUkm reactions. Water dissociated by UV produced O,which may turn to ozone.Ozone is an excellent absorber for UV. shielding the planetary surface. Water,H and O are widely used in biomolecules. Water is abundant on Earth (We got a ocean of water!)
Ice is lighter than liquid water. The ice cover acts as insulator that prevents the complete freezing of a body of water from the bottom to the top. High surface tension. Organic compounds may be concentrated in small water droplets which enhanced reactions. Water dissociated by UV produced O2 which may turn to ozone. Ozone is an excellent absorber for UV, shielding the planetary surface. Water, H and O are widely used in biomolecules. Water is abundant on Earth (We got a ocean of water!). N NH2 N O O O H H H H H O P O - HO O - 水作为类地球环境中生命所需最好的溶剂