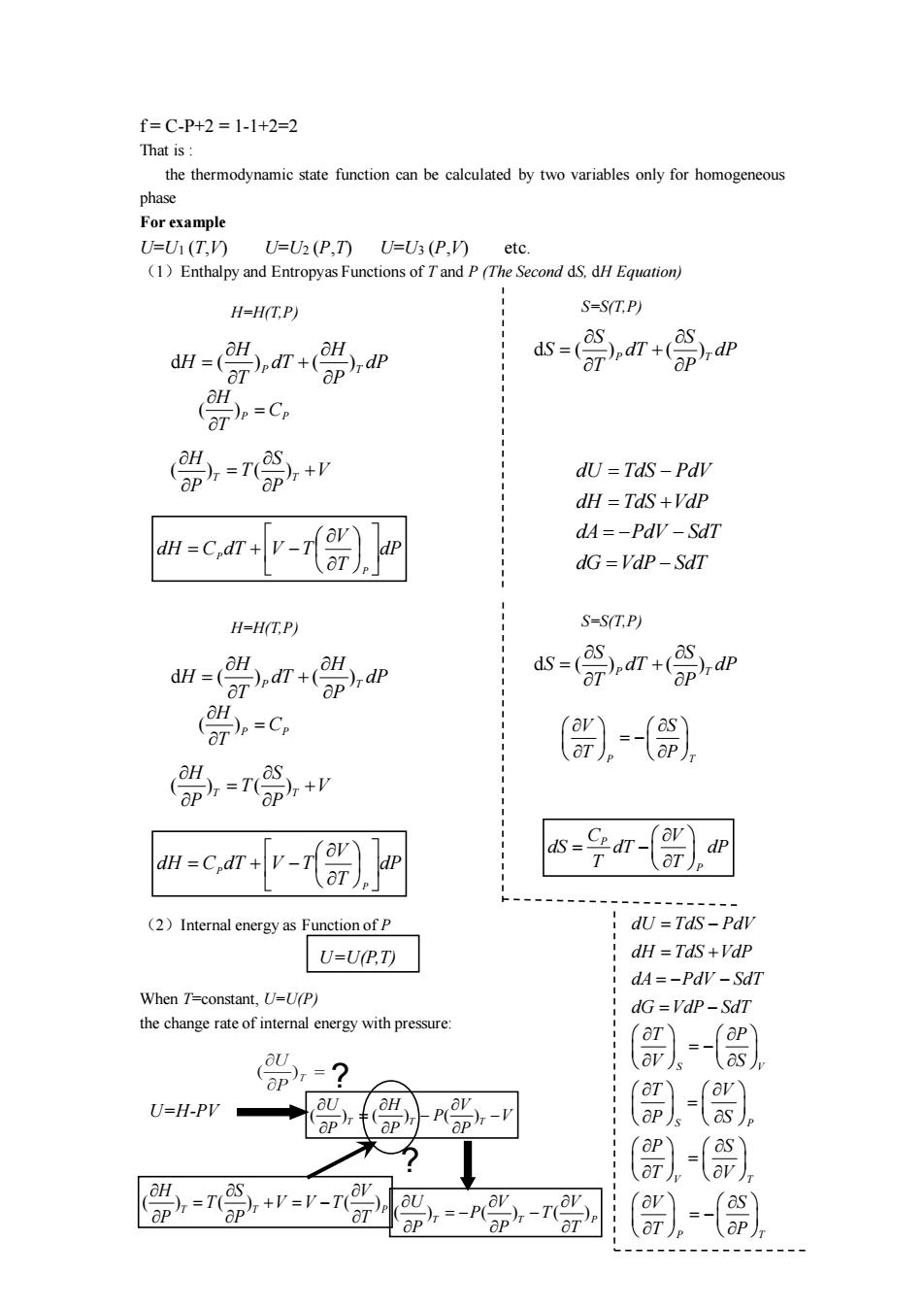
f=C-P+2=1-1+2=2 That is the thermodynamic state function can be calculated by two variables only for homogeneous phase For example U=U(T.V) UU(P n UU(P (1 Enthalpy ad Entropyas Functions Second H-H(T.P) S=S(T.P) d=欲n+0n (.c 0=n+w dU =TdS-Pdv dH TdS+VdP m-c.mv-ror) dA=-PdV-SdT dG=VdP-SdT H-H(T.P) S=S(T.P) n (,dr(),dp dS-(dp 0=C, , 0-n+w w.civ-) (2)Internal energy as Function of P dU=TdS-Pdl U=UP.T) dH =TdS+Vdp dA=-PdV-Sdl When T=constant,U-U(P) dG=VdP-SdT the change rate of interal energy with pressure 02 U=H-PV -】 -) .r-n P=-P-7
f = C-P+2 = 1-1+2=2 That is : the thermodynamic state function can be calculated by two variables only for homogeneous phase For example U=U1 (T,V) U=U2 (P,T) U=U3 (P,V) etc. (1)Enthalpy and Entropyas Functions of T and P (The Second dS, dH Equation) (2)Internal energy as Function of P When T=constant, U=U(P) the change rate of internal energy with pressure: H=H(T,P) S=S(T,P) d ( ) ( ) P T H H H dT dP T P = + d ( ) ( ) P T S S S dT dP T P = + ( )P P H C T = ( ) ( ) T T H S T V P P = + dP T V dH C dT V T P P = + − dU TdS PdV dH TdS VdP dA PdV SdT dG VdP SdT = − = + = − − = − H=H(T,P) S=S(T,P) d ( ) ( ) P T H H H dT dP T P = + d ( ) ( ) P T S S S dT dP T P = + ( )P P H C T = ( ) ( ) T T H S T V P P = + dP T V dH C dT V T P P = + − P T V S T P = − P P C V dS dT dP T T = − U=U(P,T) dU TdS PdV dH TdS VdP dA PdV SdT dG VdP SdT = − = + = − − = − S P T V T S P V P S V S T P T V P SV P V S T T = − = = − = ( ) ( ) ( ) T T P H S V T V V T P P T = + = − ( ) ( ) ( ) T T P U V V P T P P T = − − ? U=H-PV ? ( ) ( ) ( ) T T T U H V P V P P P = − −
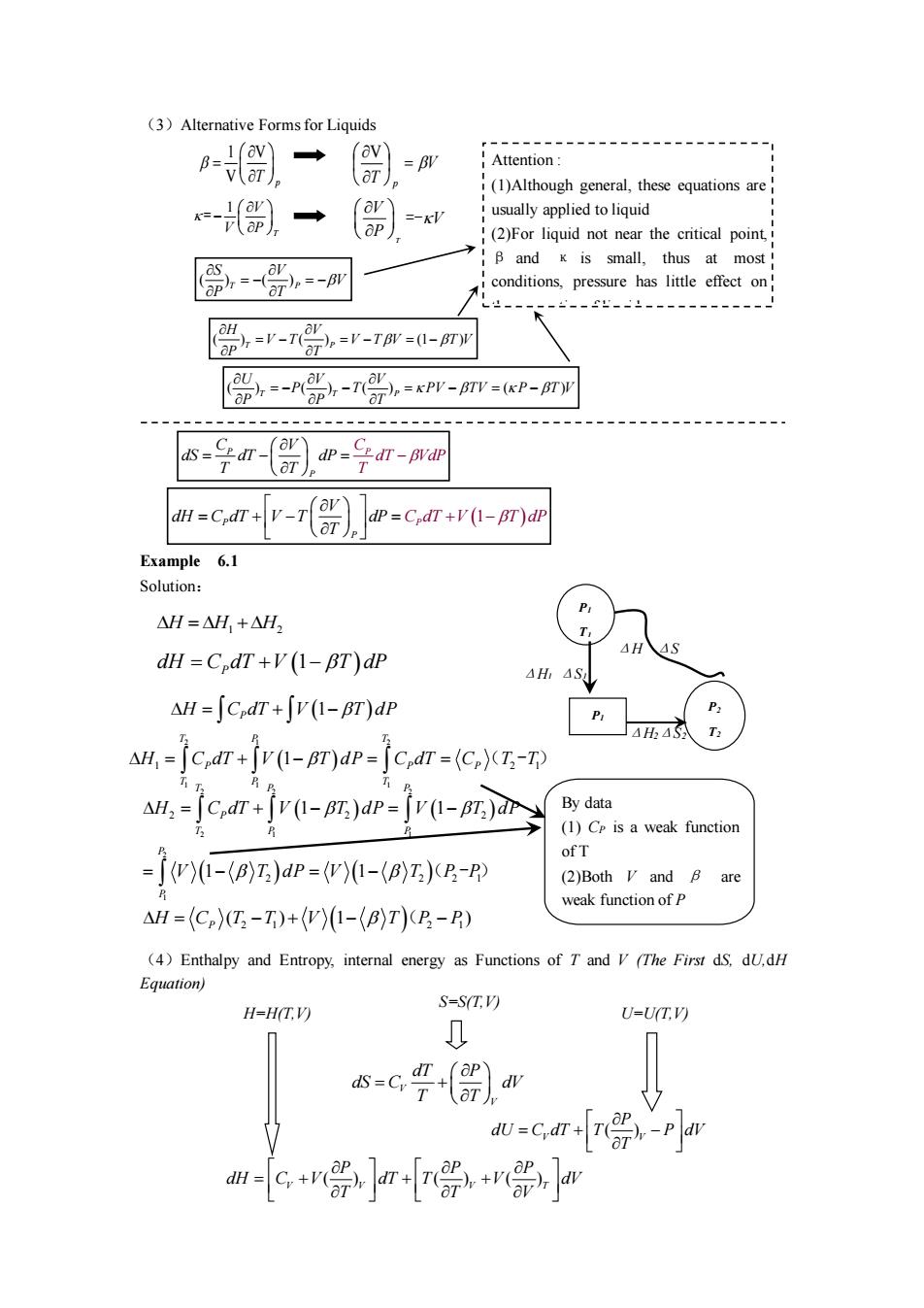
(3)Alternative Forms for Liquids (av) =BV Attention (1)Although general,these equations are -别一 usually applied to liquid (2)For liquid not near the critical point. B and x is small.thus at most 党 conditions.pressure has little effect on =-=-Tm=-m =-A0-0=Pr-mr=P-m 号r-,即号r-m dP=C,dT+v(1-BT)d Example 6.1 Solution: △H=H,+△H, dH=C dT+V(1-BT)dP AH,AS △H=∫cdr+jv(I-BT)dr 4h4 AH,=[C,dT+v(1-BT)dP=[C,dT=(C)(T-T) AH,-jc,d+jv(1-BT)adp=fv(-BT)r By data (1)C is a weak function -了y-(Bz)dP=w-z)水R-P (2)Both V and 6 are △H=(C)I,-T)+(W)1-(E)T)B-R) weak function ofp ()Enthalpy and Entropy,internal energy as Functions of T and V(The First dS.d.dH Equation) H=H(TV) S=S(T.V) U=U(T.V) s-c() =cr+r号pr m-crw
(3)Alternative Forms for Liquids Example 6.1 Solution: (4)Enthalpy and Entropy, internal energy as Functions of T and V (The First dS, dU,dH Equation) ( ) ( ) T P V P T V S − = = − ( ) ( ) (1 ) T P H V V T V T V P T T V = − = − = − ( ) ) ( ) ( ( ) T T P U V V P T PV TV P T V P P T = − − = − = − Attention : (1)Although general, these equations are usually applied to liquid (2)For liquid not near the critical point, β and κ is small, thus at most conditions, pressure has little effect on the properties of liquid 1 V V T p = V T p = V 1 T V V P − = T V V P =- P P P C dT V C V dS dT dP T T dP T = − = − P (1 ) P P V dH C dT V T dP T C dT V T dP = + − = + − P1 T1 H1 S1 P2 T2 H2 S2 ΔH ΔS P1 T2 ΔH2ΔS2 ΔH1 ΔS1 = + H H H 1 2 dH = C dT T P P + − V (1 ) d = + − H C dT V T dP P (1 ) ( ) 2 1 1 1 2 1 1 1 2 1 T P P T P T P T = − H C dT V T d + P = C dT = CP T T ( - ) ( ) ( ) ( ) ( ) 2 1 2 2 2 2 1 1 2 2 2 2 2 2 1 1 1 1 1 T P P P P T P P P V T dP V H C T dP V T P P dT V T dP = + = − = − − = − ( - ) = − + − − H C T T V T P P P ( ) 1 ) 2 1 2 1 ( () By data (1) CP is a weak function of T (2)Both V and β are weak function of P H=H(T,V) U=U(T,V) S=S(T,V) V V dT P dS C dV T T = + ( ) ( ) ( ) V V V T P P P dH C V dT T V dV T T V = + + + ( ) V V P dU C dT T P dV T = + −