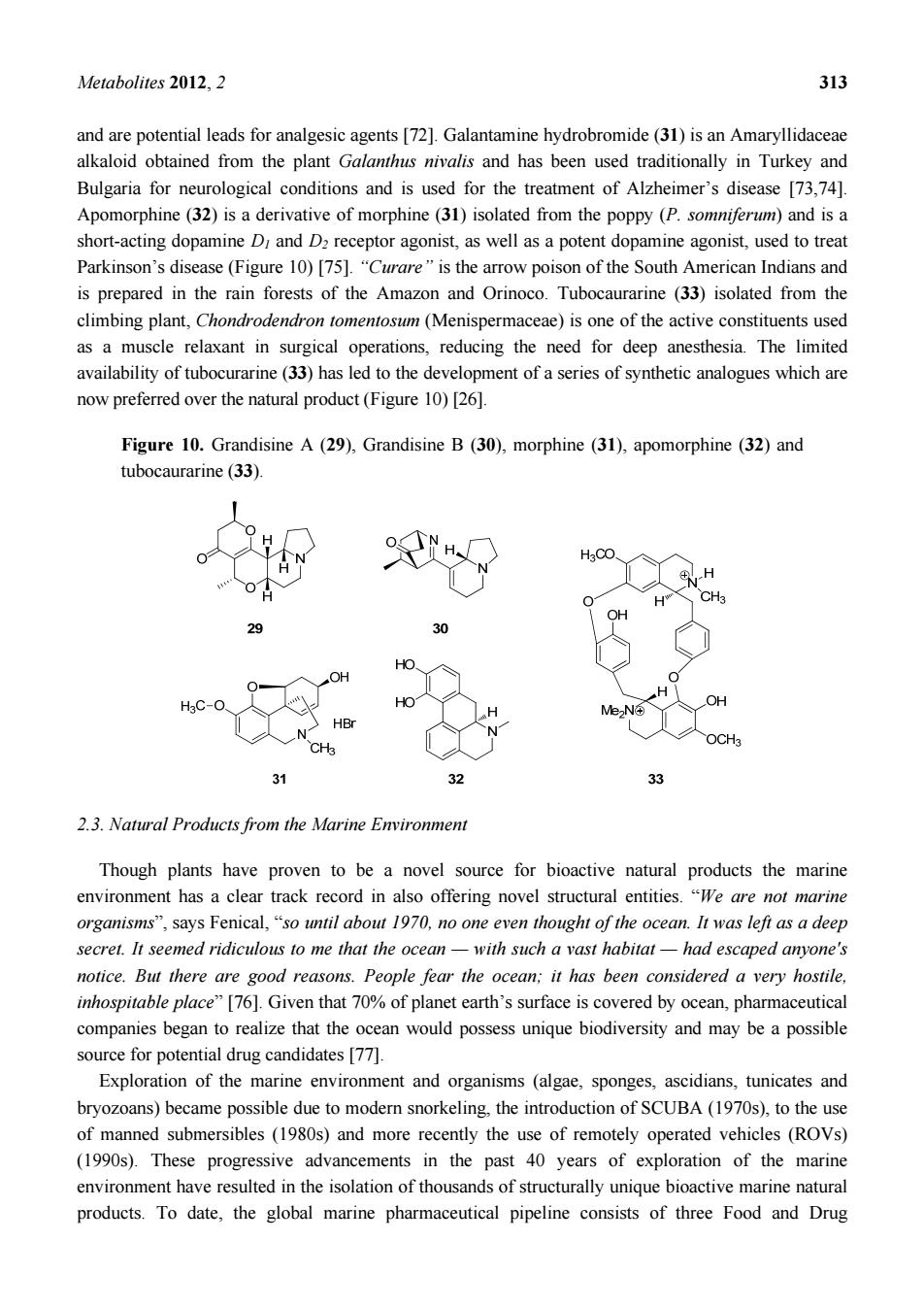
Metabolites 2012.2 313 and are potential leads for analgesic agents [72].Galantamine hydrobromide(31)is an Amaryllidaceae lkaloid obtained from the plant nivalis and has been used traditionally in Turkey and Bulgaria for neurological conditions and is used for the treatment of Alzheimer's disease [73.741 Apomorphine (32)is a derivative of morphine (31)isolated from the poppy(P.somniferum)and is a short-acting dopamine D and D receptor agonist,as well as a potent dopamine agonist,used to trea Parkinson's disease (Figure 10)[75]."Curare"is the arrow poison of the South American Indians and is prepared in the rain forests of the Amazon and Orinoco.Tubocaurarine (33)isolated from the climbing plant,Chondrodendron tomentosum (Menispermaceae)is one of the active constituents used as a muscle relaxant in surgical operations,reducing the need for deep anesthesia.The limited availability of tubocurarine(33)has led to the development of a series of synthetic analogues which are now preferred over the natural product(Figure 10)[26]. Figure 10.Grandisine A(29),Grandisine B(30),morphine (31),apomorphine (32)and tubocaurarine(33). 0 29 30 OH HO H6C- HO H Me U人N 31 32 名 2.3.Natural Products from the Marine Environment Though plants have proven to be a novel source for bioactive natural products the marine environment has a clear track record in also offering novel structural entities."We are not marine organisms",says Fenical,"so until about 1970,no one even thought of the ocean.It was left as a deep secret.It seemed ridiculous to me that the ocean -with such a vast habitat-had escaped anyone's notice.But there are good reasons.People fear the ocean:it has been considered a very hostile. inhospitable place"[76].Given that 70%of planet earth's surface is covered by ocean,pharmaceutical companies began to realize that the ocean would possess unique biodiversity and may be a possible source for potential drug candidates 1771 Exploration of the marine environment and organisms (algae,sponges,ascidians,tunicates and bryozoans)became possible due to modern snorkeling,the introduction of SCUBA(1970s),to the use of manned submersibles (1980s)and more recently the use of remotely operated vehicles (ROVs) (1990s).These progressive advancements in the past 40 years of exploration of the marin environment have resulted in the isolation of thousands of structurally unique bioactive marine natural products.To date,the global marine pharmaceutical pipeline consists of three Food and Drug
Metabolites 2012, 2 313 and are potential leads for analgesic agents [72]. Galantamine hydrobromide (31) is an Amaryllidaceae alkaloid obtained from the plant Galanthus nivalis and has been used traditionally in Turkey and Bulgaria for neurological conditions and is used for the treatment of Alzheimer’s disease [73,74]. Apomorphine (32) is a derivative of morphine (31) isolated from the poppy (P. somniferum) and is a short-acting dopamine D1 and D2 receptor agonist, as well as a potent dopamine agonist, used to treat Parkinson’s disease (Figure 10) [75]. “Curare” is the arrow poison of the South American Indians and is prepared in the rain forests of the Amazon and Orinoco. Tubocaurarine (33) isolated from the climbing plant, Chondrodendron tomentosum (Menispermaceae) is one of the active constituents used as a muscle relaxant in surgical operations, reducing the need for deep anesthesia. The limited availability of tubocurarine (33) has led to the development of a series of synthetic analogues which are now preferred over the natural product (Figure 10) [26]. Figure 10. Grandisine A (29), Grandisine B (30), morphine (31), apomorphine (32) and tubocaurarine (33). 2.3. Natural Products from the Marine Environment Though plants have proven to be a novel source for bioactive natural products the marine environment has a clear track record in also offering novel structural entities. “We are not marine organisms”, says Fenical, “so until about 1970, no one even thought of the ocean. It was left as a deep secret. It seemed ridiculous to me that the ocean — with such a vast habitat — had escaped anyone's notice. But there are good reasons. People fear the ocean; it has been considered a very hostile, inhospitable place” [76]. Given that 70% of planet earth’s surface is covered by ocean, pharmaceutical companies began to realize that the ocean would possess unique biodiversity and may be a possible source for potential drug candidates [77]. Exploration of the marine environment and organisms (algae, sponges, ascidians, tunicates and bryozoans) became possible due to modern snorkeling, the introduction of SCUBA (1970s), to the use of manned submersibles (1980s) and more recently the use of remotely operated vehicles (ROVs) (1990s). These progressive advancements in the past 40 years of exploration of the marine environment have resulted in the isolation of thousands of structurally unique bioactive marine natural products. To date, the global marine pharmaceutical pipeline consists of three Food and Drug
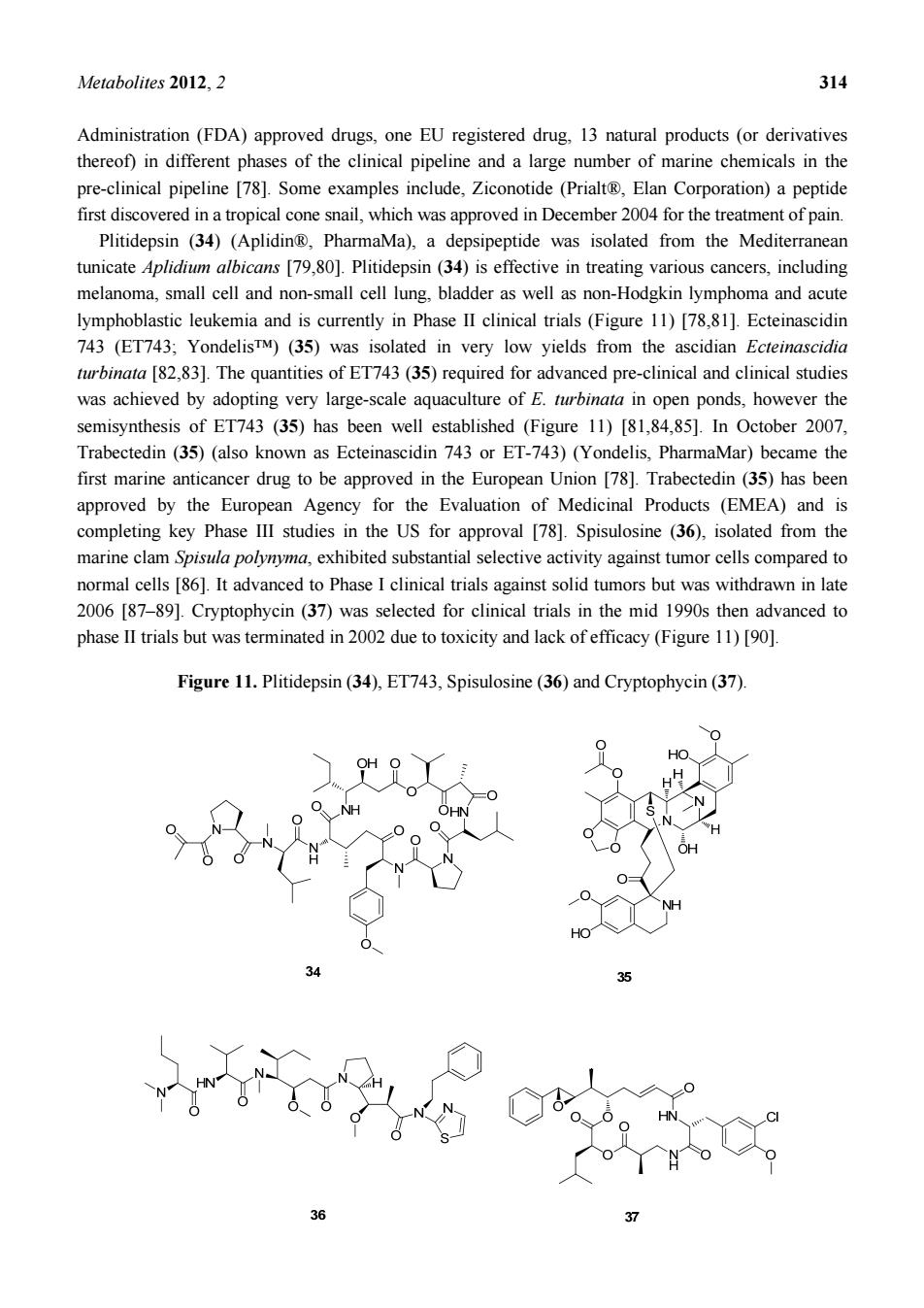
Metabolites2012.2 314 Administration(FDA)approved drugs,one EU registered drug,13 natural products (or derivatives thereof)in different phases of the clinical pipeline and a large number of marine chemicals in the pre-clinical pipeline [78].Some examples include,Ziconotide (Prialt,Elan Corporation)a peptide first discovered in a tropical cone snail,which was approved in December 2004 for the treatment of pain Plitidepsin (34)(Aplidin,PharmaMa),a depsipeptide was isolated from the Mediterranean tunicate Aplidium albicans [79,80].Plitidepsin(34)is effective in treating various cancers,including melanoma,small cell and non-small cell lung.bladder as well as non-Hodgkin lymphoma and acute lymphoblastic leukemia and is currently in Phase II clinical trials (Figure 11)[78.81].Ecteinascidin 743 (ET743:YondelisTM)(35)was isolated in very low vields from the ascidian Ecteinascidia turbinata [82,83].The quantities of ET743(35)required for advanced pre-clinical and clinical studies was achieved by adopting very large-scale aquaculture of E.turbinata in open ponds,however the semisynthesis of ET743 (35)has been well established (Figure 11)[81,84,85].In October 2007 Trabectedin (35)(also known as Ecteinascidin 743 or ET-743)(Yondelis.PharmaMar)became the first marine anticancer drug to be approved in the European Union [78].Trabectedin (35)has been approved by the European Agency for the Evaluation of Medicinal Products (EMEA)and is completing key Phase III studies in the US for approval [78].Spisulosine (36),isolated from the marine clam Spisua polynyma,exhibited substantial selective activity against tumor cells compared to normal cells [86].It advanced to Phase I clinical trials against solid tumors but was withdrawn in late 2006 [87-89].Cryptophycin (37)was selected for clinical trials in the mid 1990s then advanced to phase II trials but was terminated in 2002 due to toxicity and lack of efficacy (Figure 11)[90] Figure 11.Plitidepsin(34).ET743,Spisulosine(36)and Cryptophycin(37) OH O
Metabolites 2012, 2 314 Administration (FDA) approved drugs, one EU registered drug, 13 natural products (or derivatives thereof) in different phases of the clinical pipeline and a large number of marine chemicals in the pre-clinical pipeline [78]. Some examples include, Ziconotide (Prialt®, Elan Corporation) a peptide first discovered in a tropical cone snail, which was approved in December 2004 for the treatment of pain. Plitidepsin (34) (Aplidin®, PharmaMa), a depsipeptide was isolated from the Mediterranean tunicate Aplidium albicans [79,80]. Plitidepsin (34) is effective in treating various cancers, including melanoma, small cell and non-small cell lung, bladder as well as non-Hodgkin lymphoma and acute lymphoblastic leukemia and is currently in Phase II clinical trials (Figure 11) [78,81]. Ecteinascidin 743 (ET743; Yondelis™) (35) was isolated in very low yields from the ascidian Ecteinascidia turbinata [82,83]. The quantities of ET743 (35) required for advanced pre-clinical and clinical studies was achieved by adopting very large-scale aquaculture of E. turbinata in open ponds, however the semisynthesis of ET743 (35) has been well established (Figure 11) [81,84,85]. In October 2007, Trabectedin (35) (also known as Ecteinascidin 743 or ET-743) (Yondelis, PharmaMar) became the first marine anticancer drug to be approved in the European Union [78]. Trabectedin (35) has been approved by the European Agency for the Evaluation of Medicinal Products (EMEA) and is completing key Phase III studies in the US for approval [78]. Spisulosine (36), isolated from the marine clam Spisula polynyma, exhibited substantial selective activity against tumor cells compared to normal cells [86]. It advanced to Phase I clinical trials against solid tumors but was withdrawn in late 2006 [87–89]. Cryptophycin (37) was selected for clinical trials in the mid 1990s then advanced to phase II trials but was terminated in 2002 due to toxicity and lack of efficacy (Figure 11) [90]. Figure 11. Plitidepsin (34), ET743, Spisulosine (36) and Cryptophycin (37)