
河北医科大学药学院 Harvard University d of age.in 195 Woodward's achievements in the synthetic organic chemistry 天然药物化学教研室
河北医科大学药学院 天然药物化学教研室 6 11 Harvard University Woodward became a professor at 33 years old of age, in 1950. 12 Woodward`s achievements in the synthetic organic chemistry
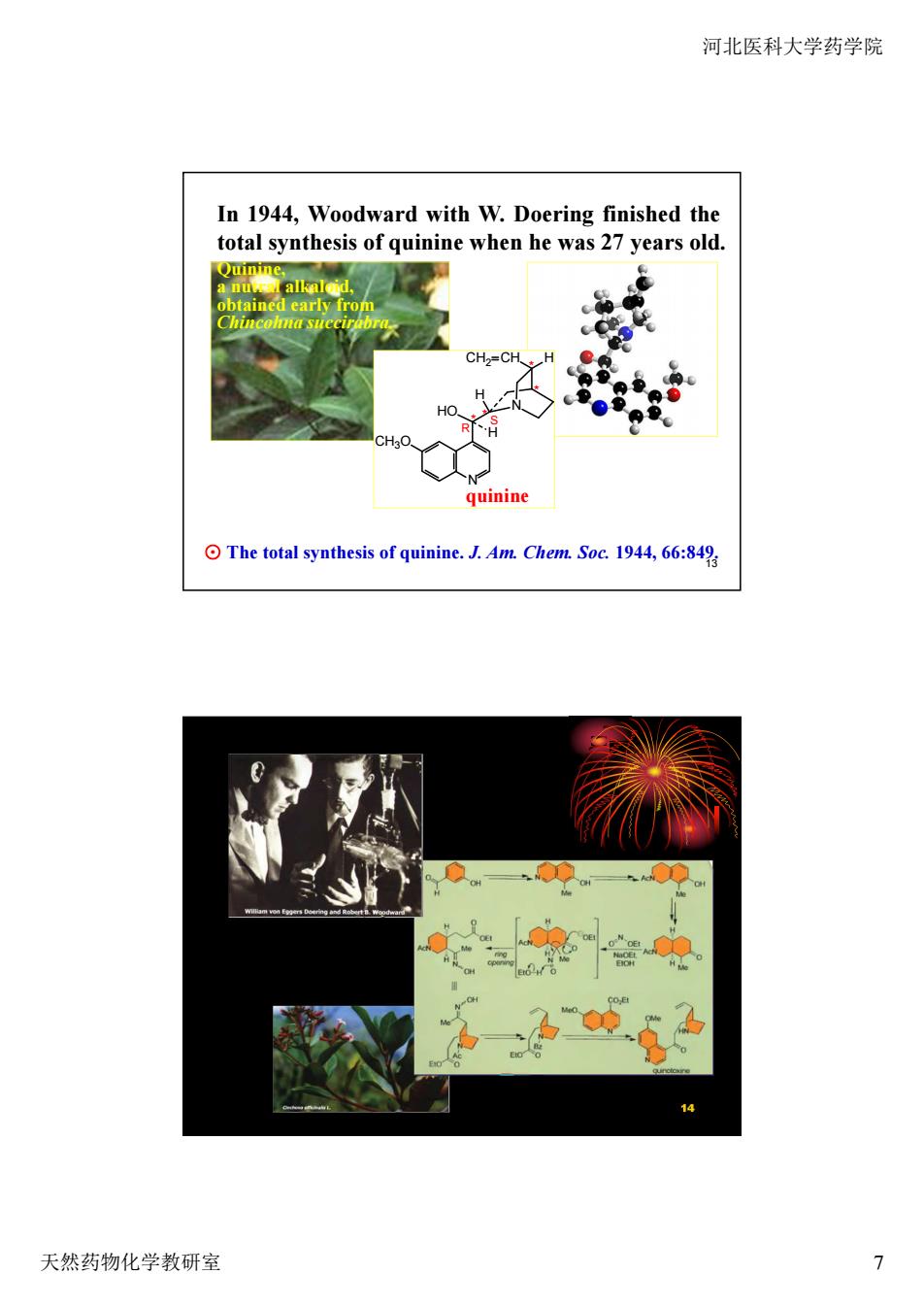
河北医科大学药学院 In 1944,Woodward with W.Doering finished the total synthesis of quinine when he was 27 years old 四 CH.=CH quinine The total synthesis of quinine.J.Am.Chem.Soc.1944,66:849 14 天然药物化学教研室 7
河北医科大学药学院 天然药物化学教研室 7 13 In 1944, Woodward with W. Doering finished the total synthesis of quinine when he was 27 years old. ⊙ The total synthesis of quinine. J. Am. Chem. Soc. 1944, 66:849. Quinine, a nutral alkaloid, obtained early from Chincohna succirabra. * * * * CH3O N HO N CH2 CH H H H quinine R S 14
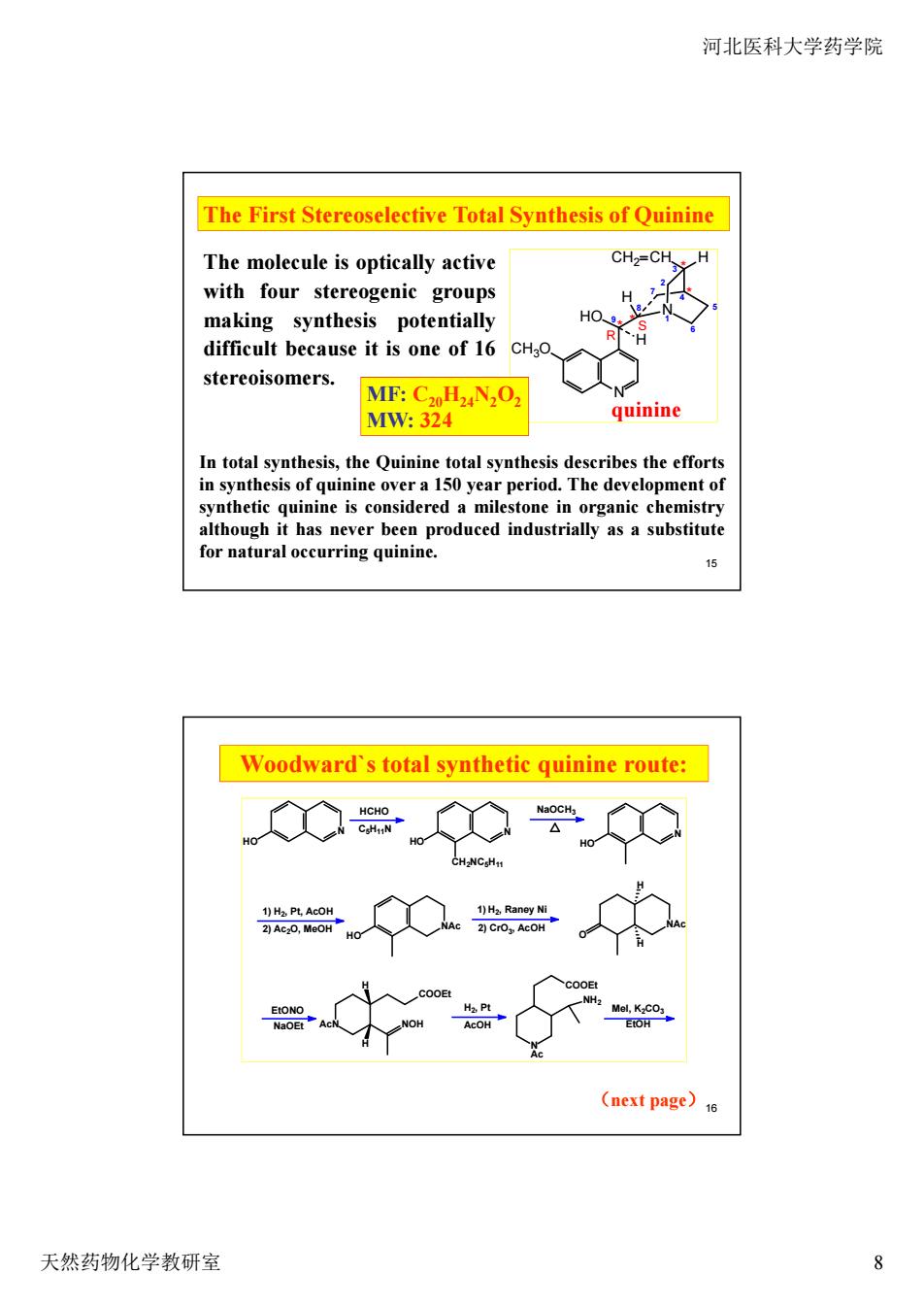
河北医科大学药学院 The First Stereoselective Total Synthesis of Quinine The molecule is optically active CHz=CH. H with four stereogenic groups making synthesis potentially difficult because it is one of 16 CH3o stereoisomers. MF:C2oHzN2Oz MW:324 quinine in fotal synthtsis,the n nine is strially as a substitute or natural occurring quin Woodward's total synthetic quinine route: K0常C学0 器÷0器÷ ÷ (next page)1 天然药物化学教研室 8
河北医科大学药学院 天然药物化学教研室 8 15 * * * * CH3O N HO N CH2 CH H H H quinine R S 1 2 3 4 5 6 7 8 9 The First Stereoselective Total Synthesis of Quinine In total synthesis, the Quinine total synthesis describes the efforts in synthesis of quinine over a 150 year period. The development of synthetic quinine is considered a milestone in organic chemistry although it has never been produced industrially as a substitute for natural occurring quinine. The molecule is optically active with four stereogenic groups making synthesis potentially difficult because it is one of 16 stereoisomers. MF: C20H24N2O2 MW: 324 16 N HO HCHO C5H11N N HO CH2NC5H11 NaOCH3 N HO 1) H2, Pt, AcOH 2) Ac2O, MeOH HO NAc 1) H2, Raney Ni 2) CrO3, AcOH NAc O H H EtONO NaOEt AcN NOH H H COOEt H2, Pt AcOH N COOEt NH2 Ac MeI, K2CO3 EtOH Woodward`s total synthetic quinine route: (next page)
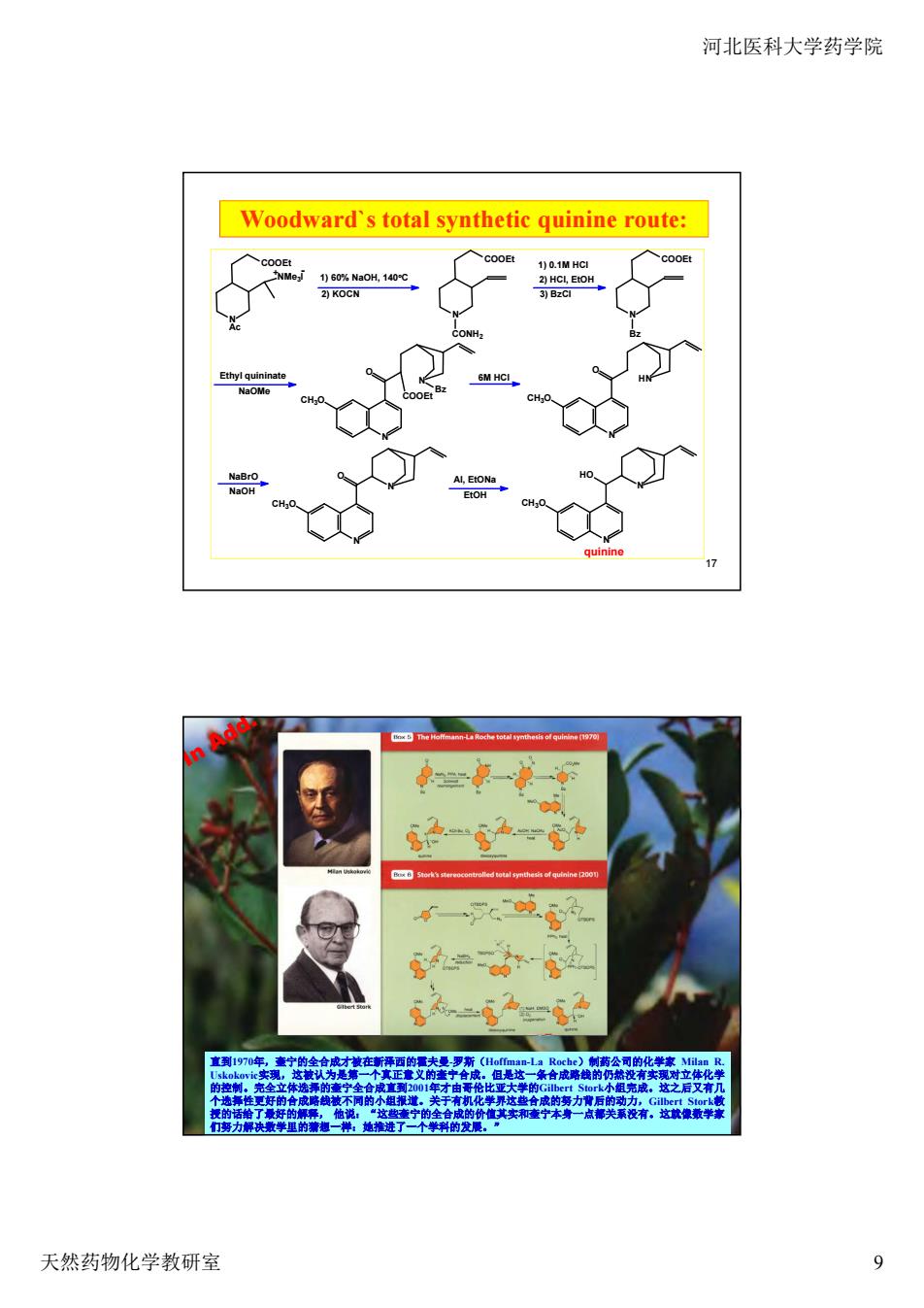
河北医科大学药学院 Woodward's total synthetic quinine route: 总一P一 6一 6学2 =g 遵制,完全 天然药物化学教研室 9
河北医科大学药学院 天然药物化学教研室 9 17 Woodward`s total synthetic quinine route: 1) 60% NaOH, 140o C 2) KOCN N COOEt NMe3I Ac + - N COOEt CONH2 1) 0.1M HCl 2) HCl, EtOH 3) BzCl N COOEt Bz Ethyl quininate NaOMe N CH3O O N COOEt Bz 6M HCl N CH3O O HN NaBrO NaOH N CH3O O N N CH3O HO N quinine Al, EtONa EtOH 18 直到1970年,奎宁的全合成才被在新泽西的霍夫曼-罗斯(Hoffman-La Roche)制药公司的化学家 Milan R. Uskokovic实现,这被认为是第一个真正意义的奎宁合成。但是这一条合成路线的仍然没有实现对立体化学 的控制。完全立体选择的奎宁全合成直到2001年才由哥伦比亚大学的Gilbert Stork小组完成。这之后又有几 个选择性更好的合成路线被不同的小组报道。关于有机化学界这些合成的努力背后的动力,Gilbert Stork教 授的话给了最好的解释, 他说:“这些奎宁的全合成的价值其实和奎宁本身一点都关系没有。这就像数学家 们努力解决数学里的猜想一样:她推进了一个学科的发展
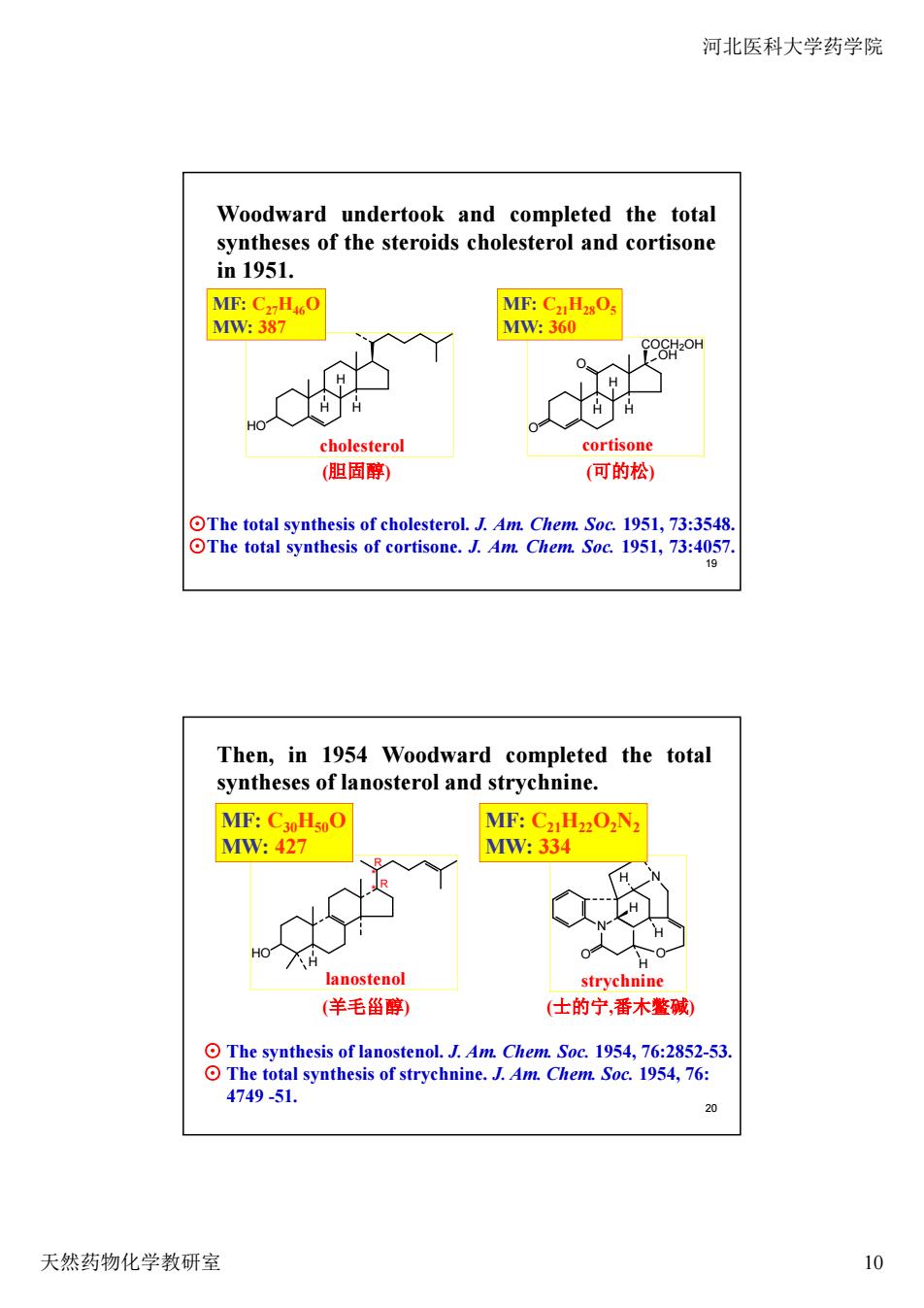
河北医科大学药学院 Woodward undertook and completed the total syntheses of the steroids cholesterol and cortisone in1951. MF:CxHO MF:CaHzOs MW:387 M:360 COCHZOH cholesterol cortisone (胆固酵) (可的松) OThe total synthesis of cholesterol.J.Am.Chem.Soc.1951,73:3548. OThe total synthesis of cortisone.J.Am.Chem.Soc.1951,73:4057. 19 Then,in 1954 Woodward completed the total syntheses of lanosterol and strychnine. MF:CxoHs0O MF:Cz.Hz2O2N2 MM:427 MW:334 H 0 lanostenol strvchnine (羊毛甾醇) 士的宁,番木碱) The synthesis of lanostenolChem Soc.1954.76:2852 al synthesis of strychnine.J.Am.Chem.Soc.1954,76: 4749-51 0 天然药物化学教研室 10
河北医科大学药学院 天然药物化学教研室 10 19 H H H O O OH COCH2OH cholesterol cortisone HO H H H Woodward undertook and completed the total syntheses of the steroids cholesterol and cortisone in 1951. ⊙The total synthesis of cholesterol. J. Am. Chem. Soc. 1951, 73:3548. ⊙The total synthesis of cortisone. J. Am. Chem. Soc. 1951, 73:4057. (胆固醇) (可的松) MF: C27H46O MW: 387 MF: C21H28O5 MW: 360 20 N O O N H H H H strychnine * * HO H R R lanostenol Then, in 1954 Woodward completed the total syntheses of lanosterol and strychnine. ⊙ The synthesis of lanostenol. J. Am. Chem. Soc. 1954, 76:2852-53. ⊙ The total synthesis of strychnine. J. Am. Chem. Soc. 1954, 76: 4749 -51. (羊毛甾醇) (士的宁,番木鳖碱) MF: C30H50O MW: 427 MF: C21H22O2N2 MW: 334