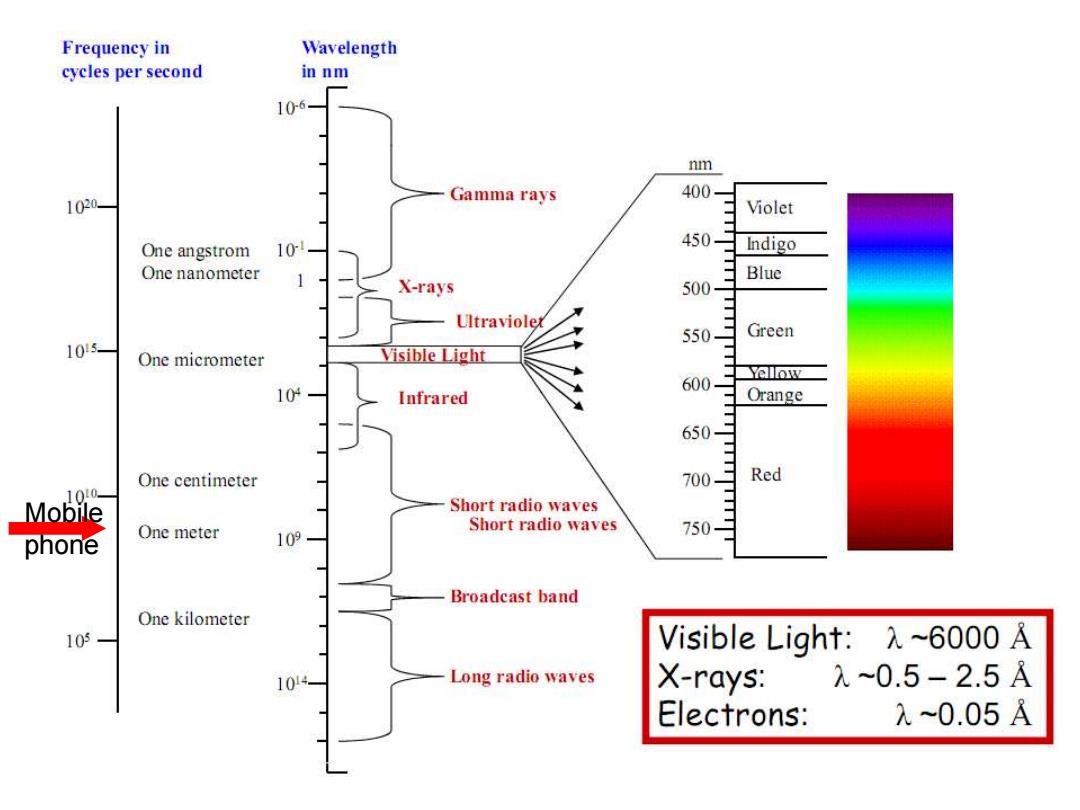
Frequency in Wavelength cycles per second in nm 106 m 400- 1020 Gamma rays Violet One angstrom 10- 450 Indigo One nanometer Blue X-rays 500- Ultraviolex Green 105 One micrometer Visible Light Yellow 104 Infrared Orange 人无令 One centimeter Red 100- Mobile Short radio waves phone One meter Short radio waves 109 Broadeast band One kilometer 105 Visible Light:X~6000 A 10 Long radio waves X-rays: λ0.5-2.5A Electrons: 元~0.05A
6 Mobile phone
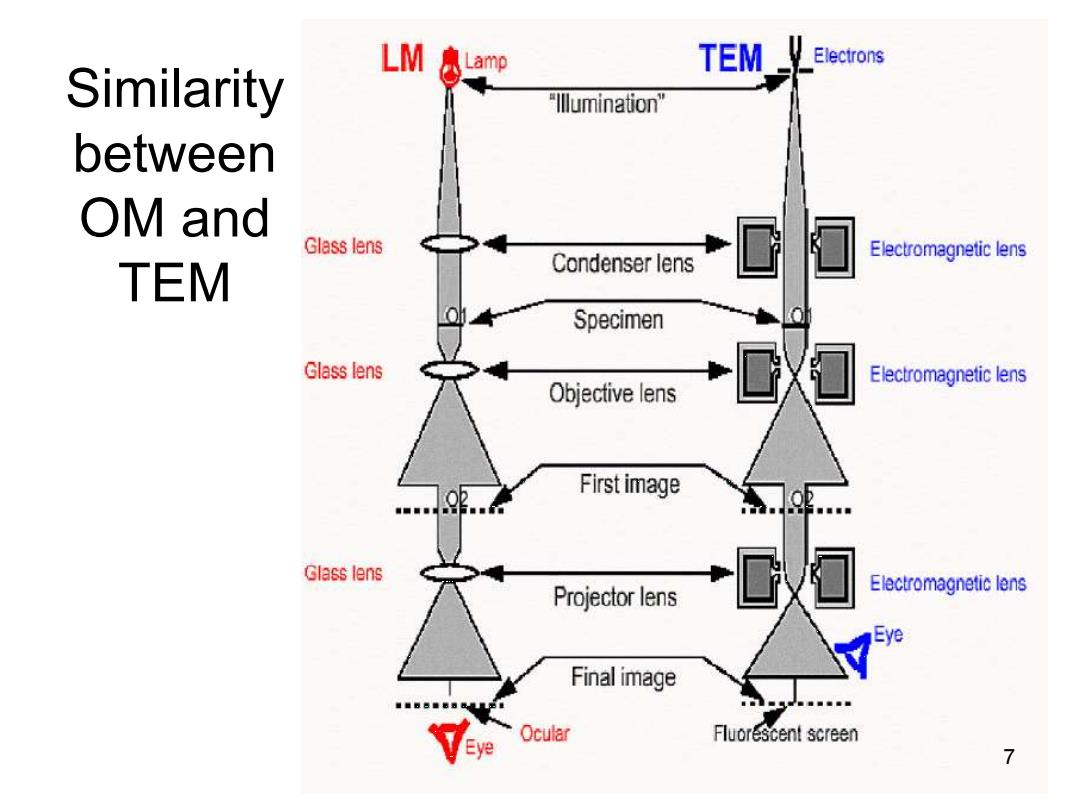
LM Lamp TEM Electrons Similarity "lllumination" between OM and Glass lens Electromagnetic lens TEM Condenser lens Specimen Glass lens Electromagnetic lens Objective lens First image Glass lens Projector lens Electromagnetic lens Final image Ocular Fluorescent screen Eye 7
Similarity between OM and TEM 7
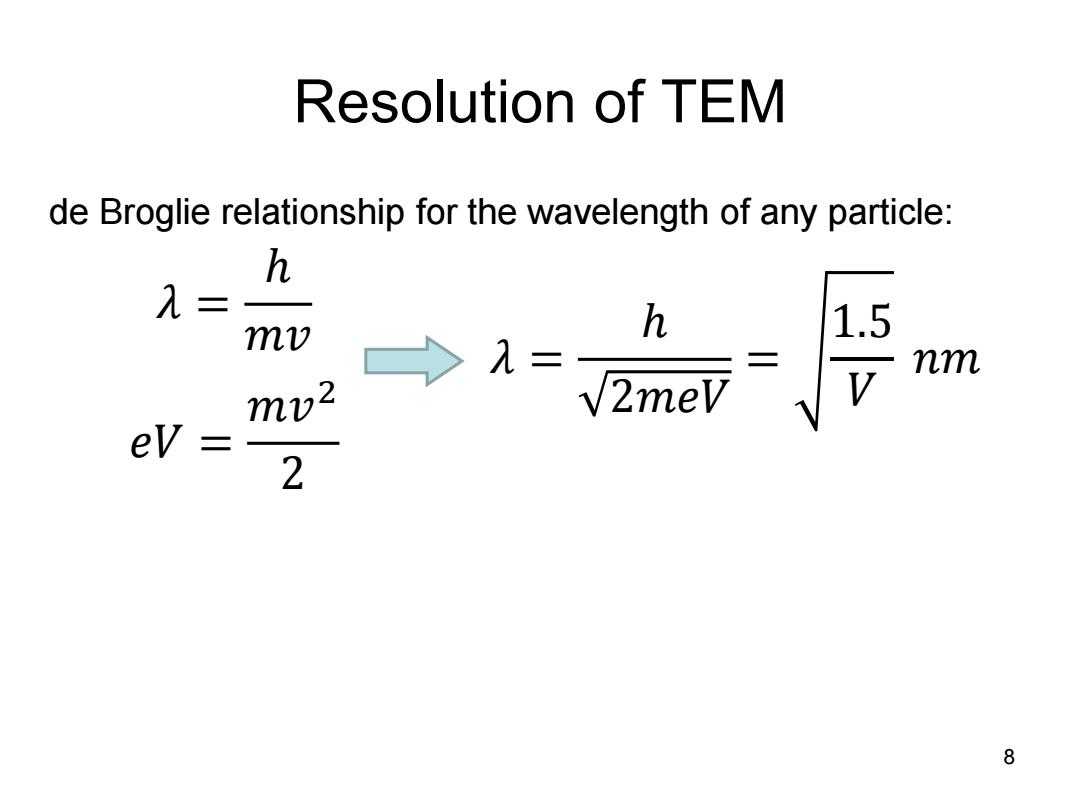
Resolution of TEM de Broglie relationship for the wavelength of any particle: h λ= mv h 1.5 >1=、 2meV 三 nm mv2 ev 二 2 8
Resolution of TEM 8 de Broglie relationship for the wavelength of any particle: 𝜆 = ℎ 𝑚𝑣 𝑒𝑉 = 𝑚𝑣 2 2 𝜆 = ℎ 2𝑚𝑒𝑉 = 1.5 𝑉 𝑛𝑚
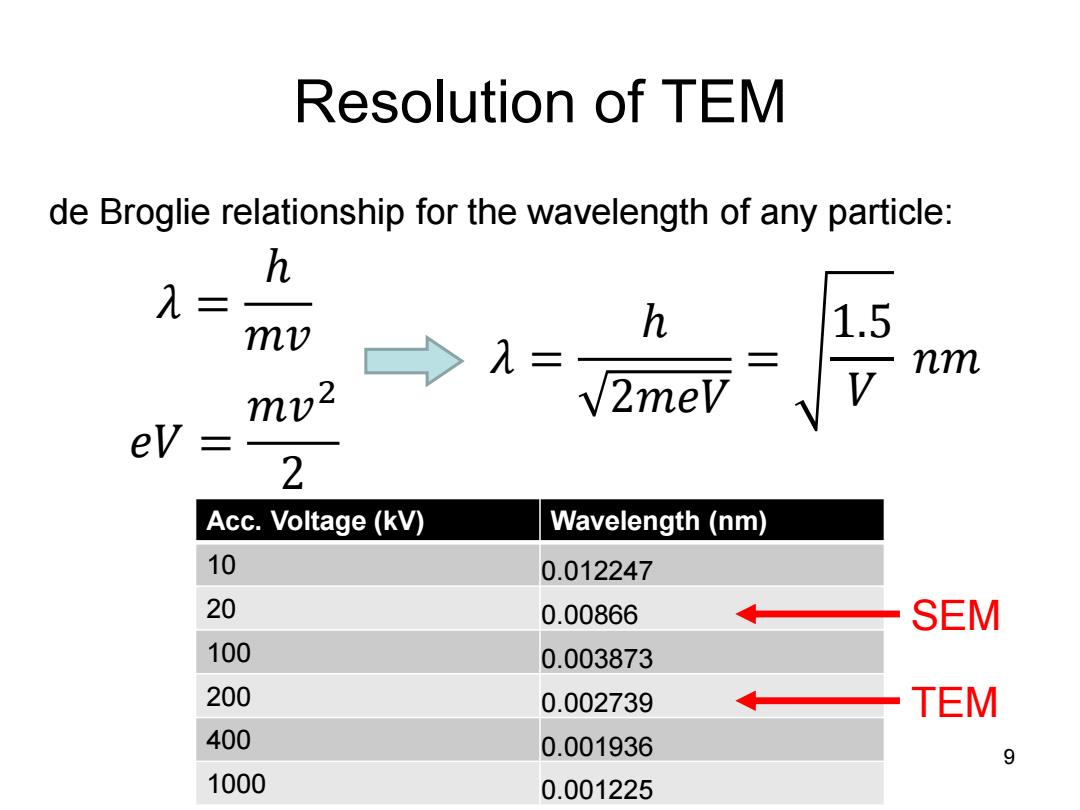
Resolution of TEM de Broglie relationship for the wavelength of any particle: h λ= mv h 1.5 。λ= 二 nm mv2 √2meV 2 Acc.Voltage(kV) Wavelength (nm) 10 0.012247 20 0.00866 SEM 100 0.003873 200 0.002739 TEM 400 0.001936 9 1000 0.001225
Resolution of TEM 9 de Broglie relationship for the wavelength of any particle: 𝜆 = ℎ 𝑚𝑣 𝑒𝑉 = 𝑚𝑣 2 2 𝜆 = ℎ 2𝑚𝑒𝑉 = 1.5 𝑉 𝑛𝑚 Acc. Voltage (kV) Wavelength (nm) 10 0.012247 20 0.00866 100 0.003873 200 0.002739 400 0.001936 1000 0.001225 SEM TEM

h λ= eV mo:rest mass of 2moeV (1+2moCZ the electron TABLE 1.2 Electron Properties as a Function of Accelerating Voltage Accelerating Non-relativistic Relativistic Mass Velocity voltage(kV) wavelength(nm)) wavelength(nm) (x mo) (×108m/s) 100 0.00386 0.00370 1.196 1.644 120 0.00352 0.00335 1.235 1.759 200 0.00273 0.00251 1.391 2.086 300 0.00223 0.00197 1.587 2.330 400 0.00193 0.00164 1.783 2.484 1000 0.00122 0.00087 2.957 2.823 TABLE 1.1 Fundamental Constants and Definitions Charge (e) (-)1.602×10-19C 1eV 1.602×1019J Rest mass(mo) 9.109×10-31kg Rest energy(moc2) 511 keV Kinetic energy (charge x voltage) 1.602 x 10-19 Nm (for 1 volt potential)=J Planck's constant(h) 6.626×1034Nms 1A 1C/s Speed of light in vacuum(c) 2.998×10m/s 10
10 𝜆 = ℎ 2𝑚0𝑒𝑉(1 + 𝑒𝑉 2𝑚0𝐶 2 ) m0 : rest mass of the electron