
The FeBr;catalyzed reaction of ethyl benzene with bromine gives the following ratio of products. CH2CH3 CH2CHs CH2CH3 CH2CH3 Br2 Br FeBra ortho meta para (38%) (<1%) (62%) The ortho and para isomers are the two major ones,whereas the meta isomer is only present in a small amount. Substituents with Nonbonding electrons The methoxyl group:Methoxybenzene (anisole)undergoes nitration around 10,000 times faster than benzene,and about 400 times faster than toluene. Since oxygen is more electronegative than carbon,it may seem strange that methoxyl is a better activating group than methyl for EAS. However,the difference is that the methoxyl group has lone pairs. Ch17 Reactions of Aromatic Compounds (landscape).docx Pagel6
Ch17 Reactions of Aromatic Compounds (landscape).docx Page16 The FeBr3 catalyzed reaction of ethyl benzene with bromine gives the following ratio of products. The ortho and para isomers are the two major ones, whereas the meta isomer is only present in a small amount. Substituents with Nonbonding electrons The methoxyl group: Methoxybenzene (anisole) undergoes nitration around 10,000 times faster than benzene, and about 400 times faster than toluene. Since oxygen is more electronegative than carbon, it may seem strange that methoxyl is a better activating group than methyl for EAS. However, the difference is that the methoxyl group has lone pairs. CH2CH3 Br2 FeBr3 CH2CH3 CH2CH3 CH2CH3 Br Br Br ortho (38%) meta (<1%) para (62%)

The lone pair can be used to stabilize adjacent positive charges through resonance. The second resonance structure is still a significant one since despite putting the positive charge on a more electronegative element (bad)since it has more bonds than the previous structure(good)and also carbon now has a full octet (good) This type of stabilization is called resonance stabilization. The oxygen atom is said to be resonance donating,or pi donating since it is donating electron density through a it bond in one of the resonance structures. Just like the activating alkyl groups,anisole preferentially activates the ortho and para positions. OCH3 OCH OCH OCH NO HNO H2SO NO NO2 anisole o-nitroanisole m-nitroanisole p-nitroanisole (31%) (2%) (67%) Chl7 Reactions of Aromatic Compounds (landscape).docx Pagel7
Ch17 Reactions of Aromatic Compounds (landscape).docx Page17 The lone pair can be used to stabilize adjacent positive charges through resonance. The second resonance structure is still a significant one since despite putting the positive charge on a more electronegative element (bad) since it has more bonds than the previous structure (good) and also carbon now has a full octet (good). This type of stabilization is called resonance stabilization. The oxygen atom is said to be resonance donating, or pi donating since it is donating electron density through a bond in one of the resonance structures. Just like the activating alkyl groups, anisole preferentially activates the ortho and para positions
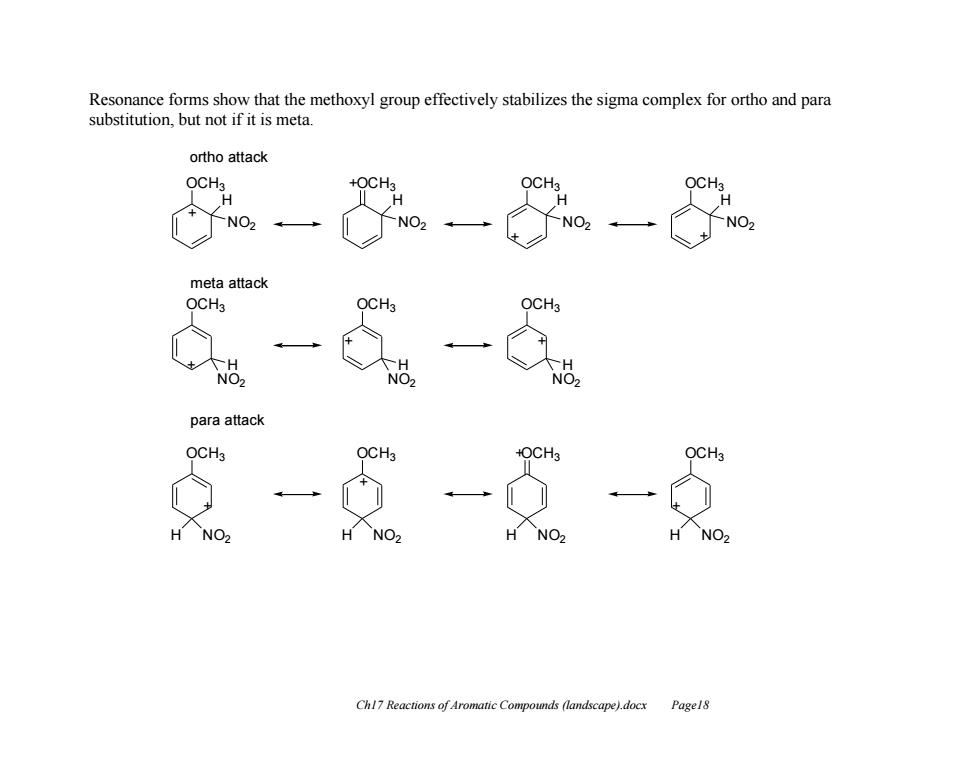
Resonance forms show that the methoxyl group effectively stabilizes the sigma complex for ortho and para substitution,but not if it is meta. ortho attack OCHa +OCH OCH OCH H NO2 meta attack OCH3 OCH3 OCH3 H TH NO2 NO2 NO2 para attack OCH3 OCH3 +OCH3 OCH3 H NO2 H NO2 H NO2 H NO> Chl7 Reactions of Aromatic Compounds (landscape).docx Pagel8
Ch17 Reactions of Aromatic Compounds (landscape).docx Page18 Resonance forms show that the methoxyl group effectively stabilizes the sigma complex for ortho and para substitution, but not if it is meta. OCH3 NO2 H + OCH3 NO2 H + OCH3 NO2 H + OCH3 NO2 H + ortho attack meta attack OCH3 OCH3 + OCH3 OCH3 OCH3 OCH3 + OCH3 H NO2 + + H NO2 H NO2 para attack H NO2 H NO2 H NO2 H NO2 + + +

The methoxyl group is so activating that anisole will react with bromine itself,and if excess bromine is used,the tribromide is readily generated. OCH3 OCH3 ■2 Br The Amino Group In a similar fashion,the lone pair of electrons on the nitrogen in an amino group causes the-NH2 substituent to be a powerful activating group with strong ortho and para directing effects. Aniline will react with bromine without a catalyst to generate tribromoaniline. NH2 NH2 Again it is the non bonding electrons that provide resonance stabilization of the sigma complex when the attack is ortho and para. Therefore any substituent with a lone pair of electrons on the atom directly bonded to the benzene ring can provide this resonance stabilization of the sigma complex for ortho and para attack. Chl7 Reactions of Aromatic Compounds (landscape).docx Page19
Ch17 Reactions of Aromatic Compounds (landscape).docx Page19 The methoxyl group is so activating that anisole will react with bromine itself, and if excess bromine is used, the tribromide is readily generated. The Amino Group In a similar fashion, the lone pair of electrons on the nitrogen in an amino group causes the -NH2 substituent to be a powerful activating group with strong ortho and para directing effects. Aniline will react with bromine without a catalyst to generate tribromoaniline. Again it is the non bonding electrons that provide resonance stabilization of the sigma complex when the attack is ortho and para. Therefore any substituent with a lone pair of electrons on the atom directly bonded to the benzene ring can provide this resonance stabilization of the sigma complex for ortho and para attack
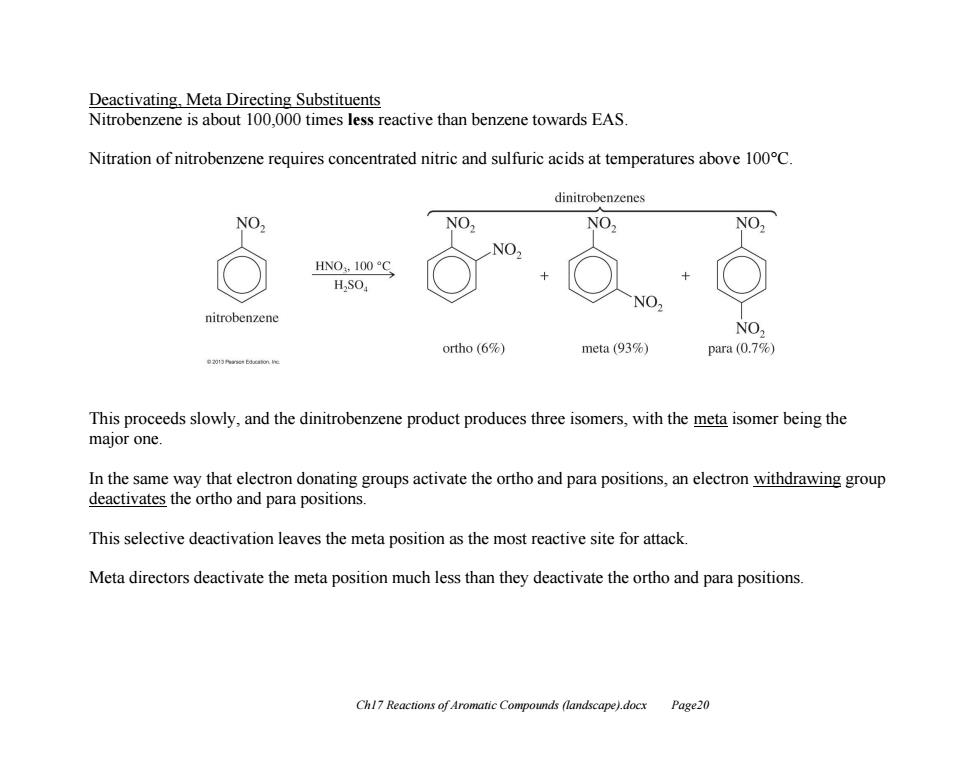
Deactivating,Meta Directing Substituents Nitrobenzene is about 100,000 times less reactive than benzene towards EAS. Nitration of nitrobenzene requires concentrated nitric and sulfuric acids at temperatures above 100C. dinitrobenzenes NO2 NO2 NO2 NO. HN0,100S HSO NO. nitrobenzene NO, ortho (6%) meta(93%) para(0.7%) This proceeds slowly,and the dinitrobenzene product produces three isomers,with the meta isomer being the major one In the same way that electron donating groups activate the ortho and para positions,an electron withdrawing group deactivates the ortho and para positions. This selective deactivation leaves the meta position as the most reactive site for attack. Meta directors deactivate the meta position much less than they deactivate the ortho and para positions. Chl7 Reactions of Aromatic Compounds (landscape).docx Page20
Ch17 Reactions of Aromatic Compounds (landscape).docx Page20 Deactivating, Meta Directing Substituents Nitrobenzene is about 100,000 times less reactive than benzene towards EAS. Nitration of nitrobenzene requires concentrated nitric and sulfuric acids at temperatures above 100°C. This proceeds slowly, and the dinitrobenzene product produces three isomers, with the meta isomer being the major one. In the same way that electron donating groups activate the ortho and para positions, an electron withdrawing group deactivates the ortho and para positions. This selective deactivation leaves the meta position as the most reactive site for attack. Meta directors deactivate the meta position much less than they deactivate the ortho and para positions