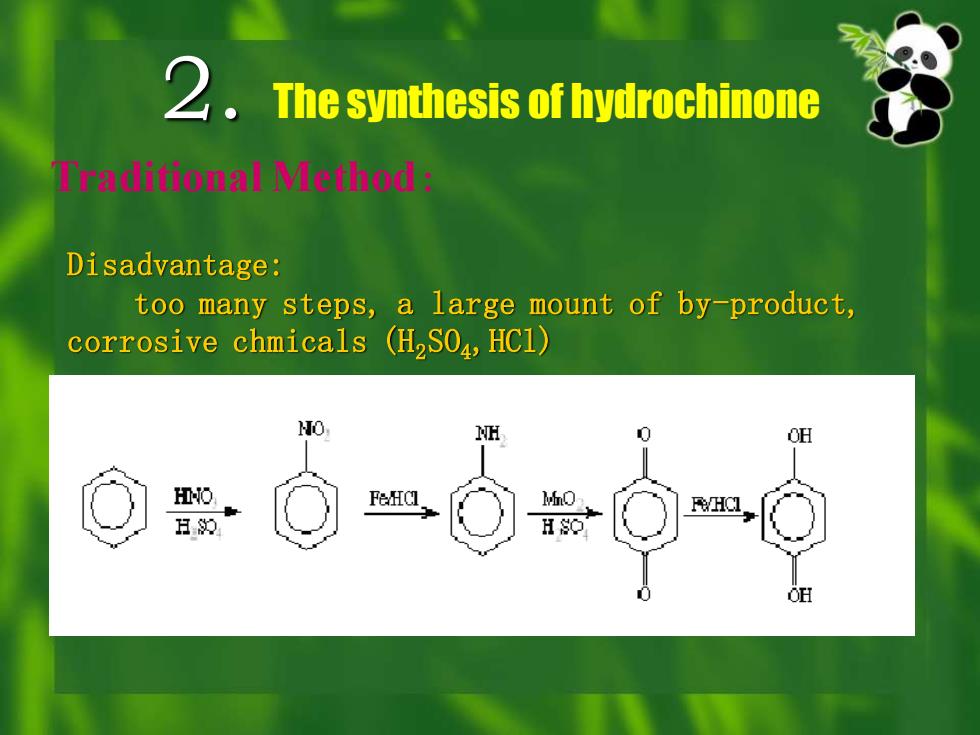
2. The synthesis of hydrochinone Traditional Method: Disadvantage: too many steps, a large mount of by-product, corrosive chmicals (H2SO4,HCl)
2. The synthesis of hydrochinone Traditional Method: Disadvantage: too many steps, a large mount of by-product, corrosive chmicals (H2SO4,HCl)
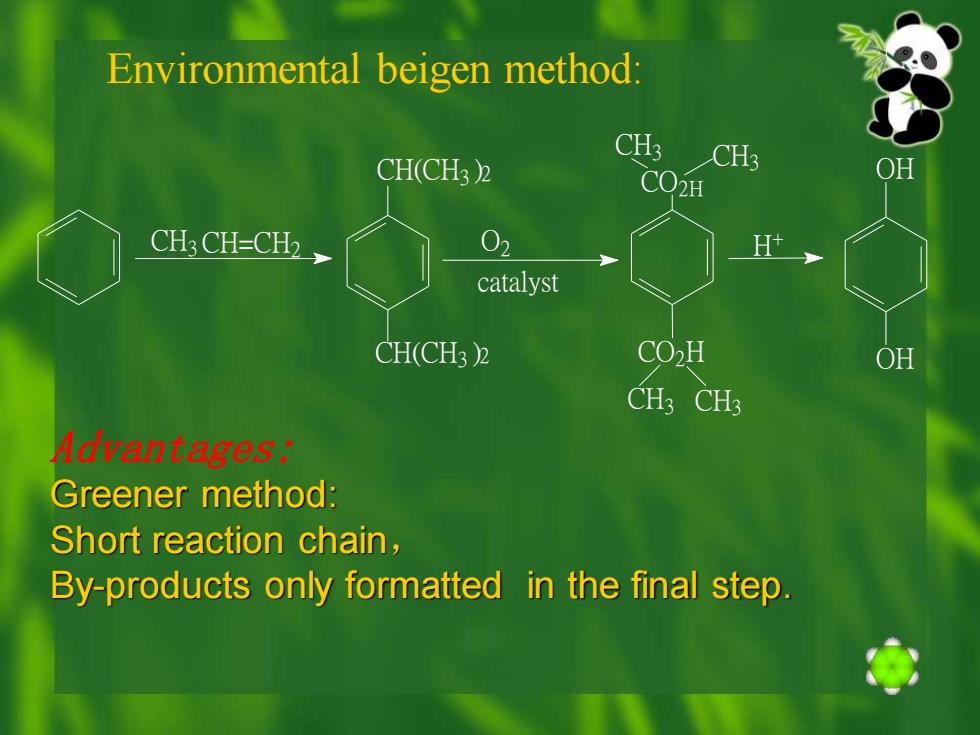
Environmental beigen method: CH3 CH3 CH3 CH3 CH3 CH3 CH=CH2 CH( )2 CH(CH3 )2 O2 CO2H CO2H OH OH H + catalyst Advantages: Greener method: Short reaction chain, By-products only formatted in the final step
Environmental beigen method: CH3 CH3 CH3 CH3 CH3 CH3 CH=CH2 CH( )2 CH(CH3 )2 O2 CO2H CO2H OH OH H + catalyst Advantages: Greener method: Short reaction chain, By-products only formatted in the final step

R1C(OH)R2 R1COR2 cat. microwave Varma et al: the activation under microwave and catalyst Traditionally Organic solvent; CrO3 , KMnO4 ; Slat pollution The synthesis of carbonyl compounds
R1C(OH)R2 R1COR2 cat. microwave Varma et al: the activation under microwave and catalyst Traditionally Organic solvent; CrO3 , KMnO4 ; Slat pollution The synthesis of carbonyl compounds
Conversion of Biomass Bamboos Small Molecules Such as: CH3COOH, CH3OH etc New Catalysts
Conversion of Biomass Bamboos Small Molecules Such as: CH3COOH, CH3OH etc New Catalysts

Chapter 5 Techniques in Green Chemistry ❖ 5.1 The performance of Catalysts in Chemical reaction ❖ 5.2 Green Chemistry and Catalysis ❖ 5.3 The Design of High Efficient and Safe Catalyst ❖ 5.4 Changing Starting Material for Chemical reaction ❖ 5.5 Changing Reagents ❖ 5.6 Changing the solvent of Chemical Reaction ❖ 5.7 Process Control and Process Intensification ❖ References
Chapter 5 Techniques in Green Chemistry ❖ 5.1 The performance of Catalysts in Chemical reaction ❖ 5.2 Green Chemistry and Catalysis ❖ 5.3 The Design of High Efficient and Safe Catalyst ❖ 5.4 Changing Starting Material for Chemical reaction ❖ 5.5 Changing Reagents ❖ 5.6 Changing the solvent of Chemical Reaction ❖ 5.7 Process Control and Process Intensification ❖ References