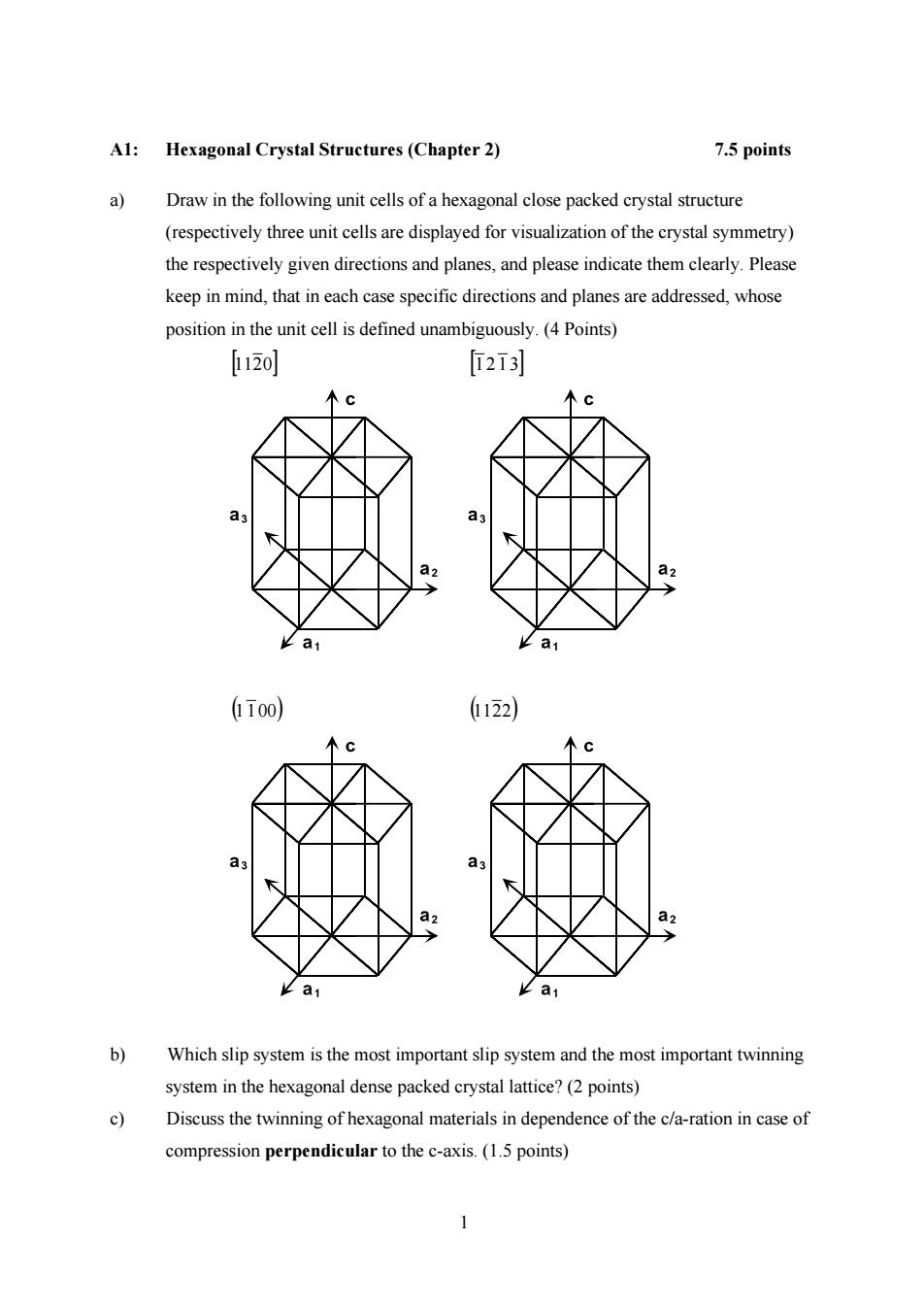
A1:Hexagonal Crystal Structures(Chapter 2) 7.5 points a) Draw in the following unit cells of a hexagonal close packed crystal structure (respectively three unit cells are displayed for visualization of the crystal symmetry) the respectively given directions and planes,and please indicate them clearly.Please keep in mind,that in each case specific directions and planes are addressed,whose position in the unit cell is defined unambiguously.(4 Points) [1120] [2i3] 木C a3 a3 a2 a2 K a1 k a1 ī00) 122) 个C a a3 a2 a2 a1 a b) Which slip system is the most important slip system and the most important twinning system in the hexagonal dense packed crystal lattice?(2 points) 9 Discuss the twinning of hexagonal materials in dependence of the c/a-ration in case of compression perpendicular to the c-axis.(1.5 points)
1 A1: Hexagonal Crystal Structures (Chapter 2) 7.5 points a) Draw in the following unit cells of a hexagonal close packed crystal structure (respectively three unit cells are displayed for visualization of the crystal symmetry) the respectively given directions and planes, and please indicate them clearly. Please keep in mind, that in each case specific directions and planes are addressed, whose position in the unit cell is defined unambiguously. (4 Points) [11 20] [1 2 1 3] (1 100) (11 22) b) Which slip system is the most important slip system and the most important twinning system in the hexagonal dense packed crystal lattice? (2 points) c) Discuss the twinning of hexagonal materials in dependence of the c/a-ration in case of compression perpendicular to the c-axis. (1.5 points)
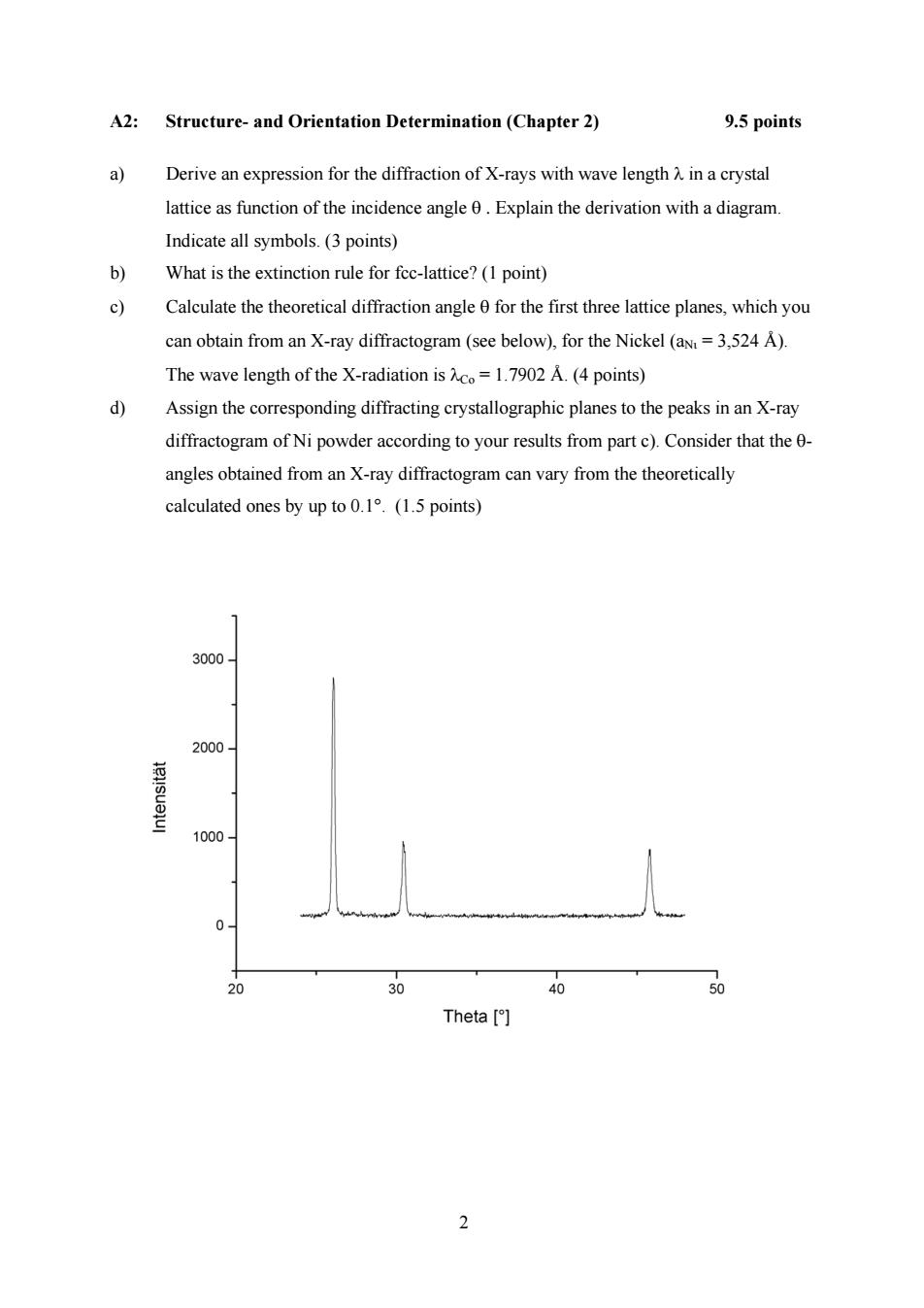
A2: Structure-and Orientation Determination(Chapter 2) 9.5 points a Derive an expression for the diffraction of X-rays with wave length A in a crystal lattice as function of the incidence angle 0.Explain the derivation with a diagram. Indicate all symbols.(3 points) b) What is the extinction rule for fcc-lattice?(1 point) c Calculate the theoretical diffraction angle 0 for the first three lattice planes,which you can obtain from an X-ray diffractogram (see below),for the Nickel (aN=3,524 A). The wave length of the X-radiation is Aco=1.7902 A.(4 points) d) Assign the corresponding diffracting crystallographic planes to the peaks in an X-ray diffractogram of Ni powder according to your results from part c).Consider that the 0- angles obtained from an X-ray diffractogram can vary from the theoretically calculated ones by up to 0.1.(1.5 points) 3000 2000- 1000- 0- 20 30 40 50 Theta [ 2
2 A2: Structure- and Orientation Determination (Chapter 2) 9.5 points a) Derive an expression for the diffraction of X-rays with wave length λ in a crystal lattice as function of the incidence angle θ . Explain the derivation with a diagram. Indicate all symbols. (3 points) b) What is the extinction rule for fcc-lattice? (1 point) c) Calculate the theoretical diffraction angle θ for the first three lattice planes, which you can obtain from an X-ray diffractogram (see below), for the Nickel (aΝι = 3,524 Å). The wave length of the X-radiation is λCo = 1.7902 Å. (4 points) d) Assign the corresponding diffracting crystallographic planes to the peaks in an X-ray diffractogram of Ni powder according to your results from part c). Consider that the θ- angles obtained from an X-ray diffractogram can vary from the theoretically calculated ones by up to 0.1°. (1.5 points)
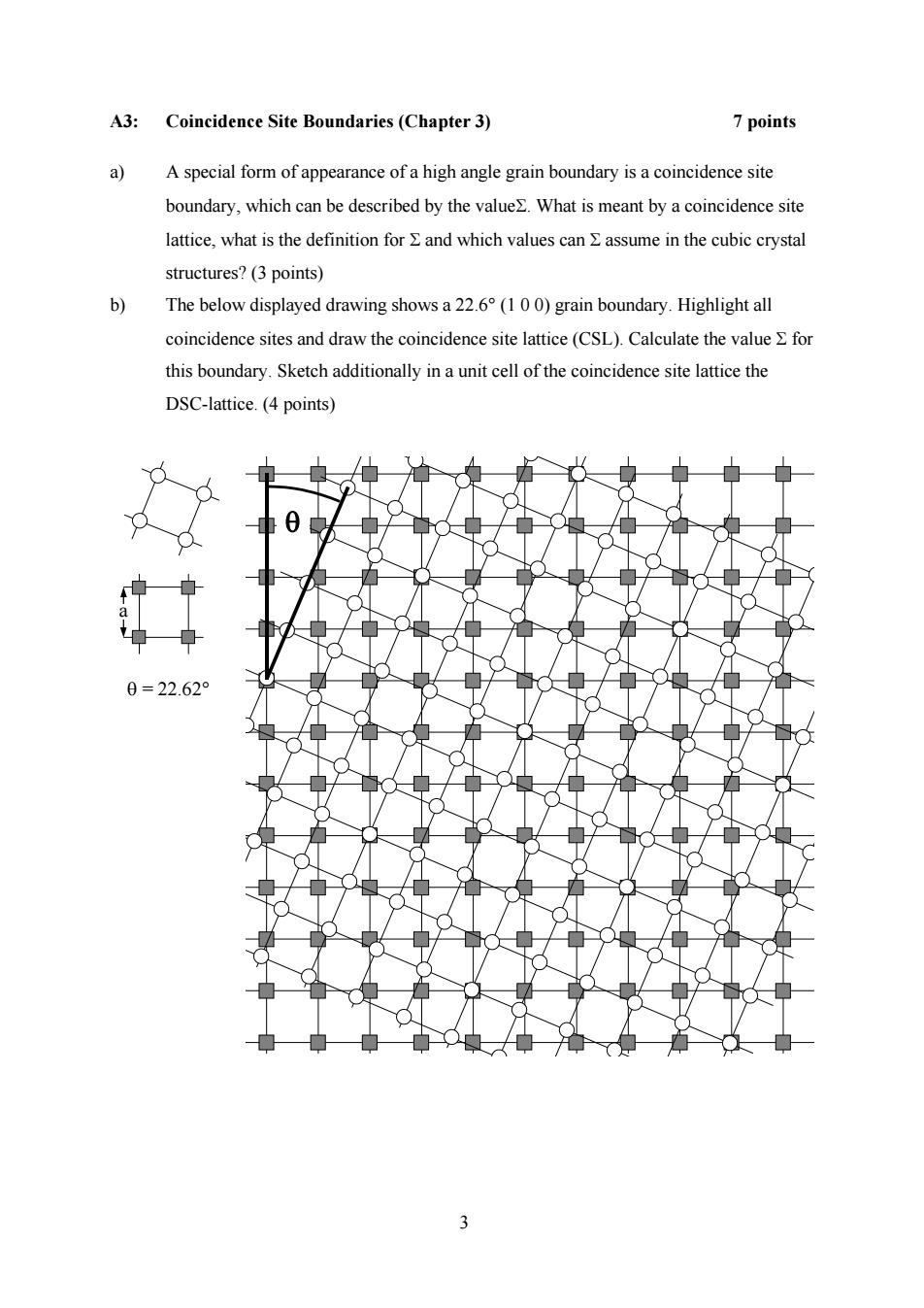
A3:Coincidence Site Boundaries(Chapter 3) 7 points a)A special form of appearance of a high angle grain boundary is a coincidence site boundary,which can be described by the value2.What is meant by a coincidence site lattice,what is the definition for E and which values can E assume in the cubic crystal structures?(3 points) b) The below displayed drawing shows a 22.6(1 00)grain boundary.Highlight all coincidence sites and draw the coincidence site lattice(CSL).Calculate the value E for this boundary.Sketch additionally in a unit cell of the coincidence site lattice the DSC-lattice.(4 points) 0 0=22.62° 3
3 A3: Coincidence Site Boundaries (Chapter 3) 7 points a) A special form of appearance of a high angle grain boundary is a coincidence site boundary, which can be described by the valueΣ. What is meant by a coincidence site lattice, what is the definition for Σ and which values can Σ assume in the cubic crystal structures? (3 points) b) The below displayed drawing shows a 22.6° (1 0 0) grain boundary. Highlight all coincidence sites and draw the coincidence site lattice (CSL). Calculate the value Σ for this boundary. Sketch additionally in a unit cell of the coincidence site lattice the DSC-lattice. (4 points) θ θ = 22.62° a

A4:Alloying(Chapter4) 16points a Give the Gibbs phase rule.Identify all variables in this relation.(2.5 points) b) Construct a qualitative phase diagram for the three phases S,a and B as function of the solute content cB from the Gibbs free energy curves given at the four temperatures TI to T4.Indicate all phase fields.(5 points) c)How much is the solubility limit at T=OK?Explain your answer by drawing curves of the enthalpy H(c),the entropy S(c)and the Gibbs free energy G(c)as a function of the solute concentration c in a graph.(2.5 points) 少 Draw the unit cell of the ordered alloy compounds CuAu and Ni3Al.(2 points) e) How is the long range order parameter s defined(equation)?Give a relation and explain the different quantities appearing in it.Which range of values does this parameter take in case of an AB alloy?And for an AB3 alloy?Account for your answer using simple calculations.(4 points)
4 A4: Alloying (Chapter4) 16points a) Give the Gibbs phase rule. Identify all variables in this relation. (2.5 points) b) Construct a qualitative phase diagram for the three phases S, α and β as function of the solute content cB from the Gibbs free energy curves given at the four temperatures T1 to T4. Indicate all phase fields. (5 points) c) How much is the solubility limit at T= 0K? Explain your answer by drawing curves of the enthalpy H(c), the entropy S(c) and the Gibbs free energy G(c) as a function of the solute concentration c in a graph. (2.5 points) d) Draw the unit cell of the ordered alloy compounds CuAu and Ni3Al. (2 points) e) How is the long range order parameter s defined (equation)? Give a relation and explain the different quantities appearing in it. Which range of values does this parameter take in case of an AB alloy? And for an AB3 alloy? Account for your answer using simple calculations. (4 points)
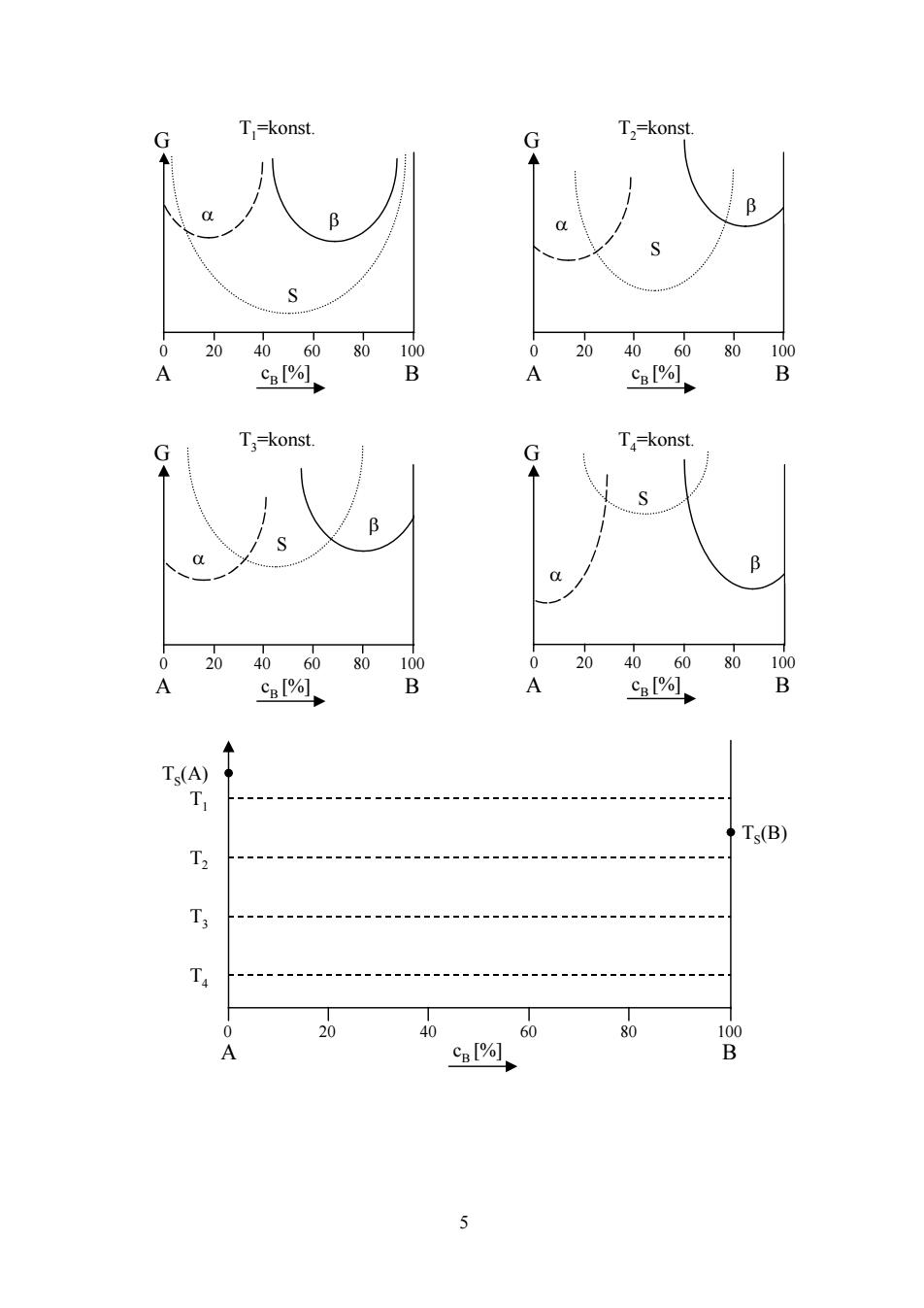
G T=konst. G T,=konst. B B S 0 2040 60 80 100 0 20 40 60 80 100 A Cal%l B A Cpl% B T=konst. G G T=konst. S B B 0 20 4060 80 100 0 20 40 6080100 A c%, B A c%, B Ts(A T Ts(B) T2 T 0 20 40 60 80 100 A CB [% B 5
5 G T3=konst. G T4=konst. S α β β S α 0 20 40 60 80 100 A B cB [%] 0 20 40 60 80 100 A B cB [%] G T1=konst. G T2=konst. S α β β α S 0 20 40 60 80 100 A B cB [%] 0 20 40 60 80 100 A B cB [%] T4 T3 T2 T1 TS(A) TS(B) 0 20 40 60 80 100 A B cB [%]