
Ig M(OH)PH 18 Ig a M(OH) 16 14 Pb 12 10 8 6 4 2 Bi 0 2 4 8101214 6 pH
0 2 4 6 8 10 12 14 16 18 0 2 4 6 8 10 12 14 pH Al FeIII Bi Zn Pb Cd Cu FeII lg α M(OH) ~pH lg α M(OH)

2个副反应同时存在时 MMH]+M(OH)]+-]+[MA] [M] [W+[MOH+M(OH)2]+.+[M+[MA]+MA2]+-[M [M] OM(OH)+M(A)-1 若有n个副反应 &M=aM(AI)+a M(A2)+-M(An)-(n-1)
2个副反应同时存在时 2 M 2 [M]+[MOH]+M(OH) ]+-[MA]+[MA ]+- = ] - [M 2 2 [M]+[MOH]+M(OH) ]+ +[M + [MA]+[MA ]+ = [M] ] -[M] = M(OH) M(A) + 1- M = M(A1)+ M(A2) +- M(An)-(n-1) 若有n个副反应

例用EDTA滴定Zn2+至化学计量点附近, pH=11.00,NH3]=0.10molL1, 计算gzn OZn(NH3) 1+[NH318+[NH312B2+[NH313 B3+NH314B =1+10-1.00+2.27+10-2.00+4.61+10-3.00+7.01+10-4.00+9.06 =+10227+102.64104.01+105.06=105.10 查附录.6,pH=11.00时,gCZn(om-5.4 Ig @zn=Ig(@Zn(NH3)+@zn(OH)-1)=1g(1051+1054-1)=5.6
例 用EDTA滴定Zn2+至化学计量点附近, pH = 11.00,[NH3 ]=0.10mol·L-1 , 计算 lg Zn Zn(NH3)= 1+[NH3 ]1+[NH3 ] 2 2+[NH3 ] 3 3+[NH3 ] 4 4 =1+10-1.00+2.27+10-2.00+4.61+10-3.00+7.01+10-4.00+9.06 =1+101.27+102.61+104.01+105.06=105.10 查附录III.6 , pH=11.00时, lg Zn(OH)=5.4 lg Zn= lg( Zn(NH3)+ Zn(OH)-1)=lg(105.1+105.4 -1)= 5.6

例用EDTA滴定Zn+至化学计量点附近, pH=9.0,cNH3)=0.10moL, 计算lg&ZmNH3) c(NH3)=[NH3l+[NH*H+ ∑Zn(NH3J ↓ [NH3'] 忽略 KaNH4)=109.37(I=0.1) @NH3(H) INH3]=1+[H*]K(NH4) [NH3] =1+109.0+9.4=100.5
例 用EDTA滴定Zn2+至化学计量点附近, pH = 9.0,c(NH3 )=0.10mol·L-1 , 计算lg Zn(NH3) c(NH3 )=[NH3 ]+[NH4 +]+ ( ) ( ) ( ) ] 3 + H + NH 4 + -9.37* a 4 3 -9.0+9.4 0. 3 H 5 [NH = = 1+[H ] NH [NH ] = 1+1 (NH ) 1 = 0. = 0 = 0 1 K 0 I 1 K 3 [Zn(NH ) ]i 3 [NH ] 忽略
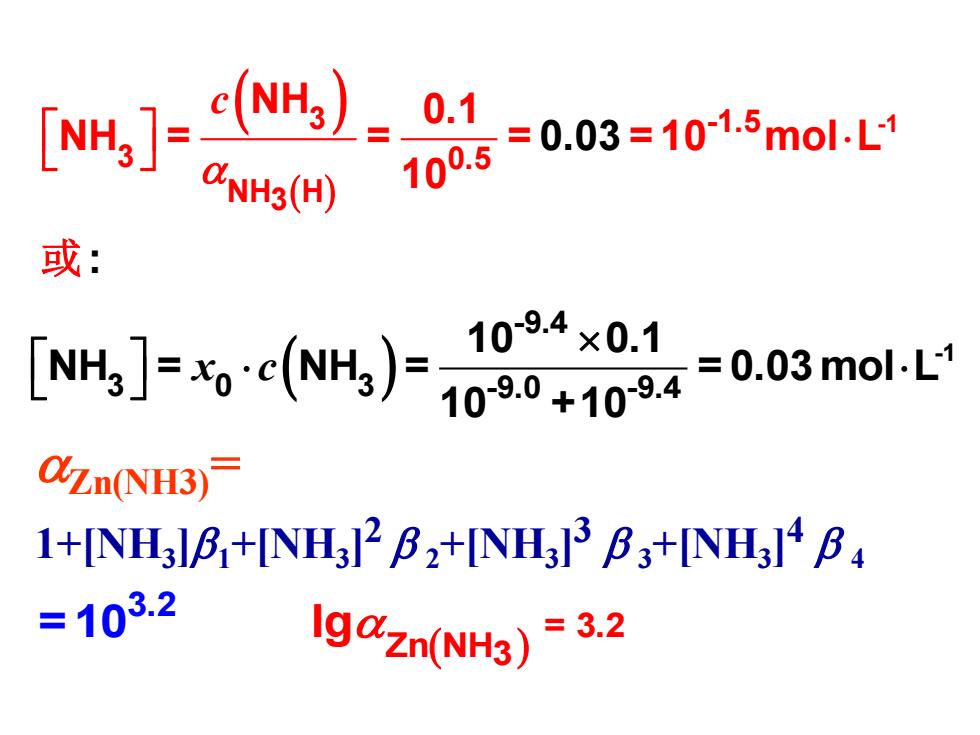
]=c) 0.1 @NH3(H) 100.5 =0.03=101.5mol-L 或: J60)00 =0.03mo.L-1 QZn(NH3) 1+[NH3l8+[NH312B2+[NH3]3B3+[NH31B =103.2 Ig@zn(NH3)=3.2
( ) ( ) 3 -1.5 -1 3 0.5 NH H 3 NH 0.1 NH = = = = 10 mol 0.03 1 : 0 L c 或 ( ) 3. = 3.2 H3 2 Zn N =10 lg Zn(NH3)= 1+[NH3 ]1+[NH3 ] 2 2+[NH3 ] 3 3+[NH3 ] 4 4 ( ) -1 -9.4 3 0 3 -9.0 -9.4 10 0.1 NH = NH = = 0.03 mol L 10 +10 x c