
centrifuged in microhematocrit tubes for 3 minutes in a clay-Adams Autocrit II/(Snieszko 1960). Blood samples for hemoglobin determination were either read directly with an A-0 hemoglobinometer or collected in 20ul capillary tubes to determine hemoglobin concentration by the calorimetric method described by Bauer (1970) Viral Assays In 1978,liver,spleen,and kidney tissues from 60 fish in each test group were sampled,pooled in 12 tubes of 5 fish each,and screened by a private laboratory (Rangen Research Laboratories)for viruses.In 1979, the tissue samples from each fish were aseptically divided into equal portions.One lot was submitted to Rangen Research Laboratories and the other to the National Fisheries Research Center.The results of these independent tests are reported in Appendix A. Histopathology sixty individually numbered fish of each test group were preserved in fixatives and submitted to Bio-Med Research Laboratories.Gill,liver, eye,kidney,thyroid,brain,and olfactory tissues were sectioned, appropriately stained,and examined for any pathologic lesions or abnormalities.See Appendix B. Bacteriological Assays The sensitive and highly specific indirect fluorescent antibody technique (IFAT)was used to diagnose latent Bacterial Kidney Disease (BKD)in hatchery populations. The individually identified fish were opened ventrally and the kidney 1/Reference to trade names does not imply endorsement by National Marine Fisheries Service,NOAA
centrifuged in microhematocrit tubes for 3 minutes in a Clay-Adams Autocrit II1/ (Snieszko 1960). Blood samples for hemoglobin determination were either read directly with an A-O hemoglobinometer or collected in 20u1 capillary tubes to determine hemoglobin concentration by the calorimetric method described by Bauer (1970). Viral Assays In 1978, liver, spleen, and kidney tissues from 60 fish in each test group were sampled, pooled in 12 tubes of 5 fish each, and screened by a private laboratory (Rangen Research Laboratories) for viruses. In 1979, the tissue samples from each fish were aseptically divided into equal portions. One lot was submitted to Rangen Research Laboratories and the other to the National Fisheries Research Center. The results of these independent tests are reported in Appendix A. Histopathology Sixty individually numbered fish of each test group were preserved in fixatives and submitted to Bio-Med Research Laboratories. Gill, liver, eye, kidney, thyroid, brain, and olfactory tissues were sectioned, appropriately stained, and examined for any pathologic lesions or abnormalities. See Appendix B. Bacteriological Assays The sensitive and highly specific indirect fluorescent antibody technique (IFAT) was used to diagnose latent Bacterial Kidney Disease (BKD) in hatchery populations. The individually identified fish were opened ventrally and the kidney -1/ Reference to trade names does not imply endorsement by National Marine Fisheries Service, NOAA. 7

exposed.Thin smears of anterior and posterior kidney tissue were made on multi-spot slides after piercing the kidney with a sterile inoculation loop.The slides were air-dried and fixed in reagent grade acetone for 10 minutes.The acetone fixed slides were stored at -20c until they were examined.Prior to the sampling season,40 positive control slides were prepared in the same manner and stored at-20C.The control slides were prepared from a clean kidney lesion from a spring chinook salmon from Carson National Salmon Hatchery that was tested and confirmed to have high numbers of BKD organisms. The IFAT for BKD was originally described by Bullock and Stuckey (1975)and later modified by G.W.Camenisch (unpublished report)of the U.S.Fish and Wildlife Service (FWS),Eastern Fish Disease Laboratory.The complete procedure used in this study is described in our previous report (Novotny and Zaugg 1979). All dead and dying fish in the seawater pens were collected daily. Each fish was opened from the vent,external and internal lesions noted, and the procedures for culturing vibriosis and other gram negative bacteria (Novotny,Harrell,and Nyegaard 1975)were followed. The postmortems were classified as follows: 1.Negative (cause of death not determined) 2.BKD (from lesions). 3.Vibrio anquillarum--serotypes 775,1669,or 7244. 4.Vibrio sp. 5.ERM (enteric redmouth). 6.Furunculosis. 7.Aeromonas hydrophilia (ex.liquefaciens)
exposed. Thin smears of anterior and posterior kidney tissue were made on multi-spot slides after piercing the kidney with a sterile inoculation loop. The slides were air-dried and fixed in reagent grade acetone for 10 minutes. The acetone fixed slides were stored at -2O°C until they were examined. Prior to the sampling season, 40 positive control slides were prepared in the same manner and stored at -2O°C. The control slides were prepared from a clean kidney lesion from a spring chinook salmon from Carson National Salmon Hatchery that was tested and confirmed to have high numbers of BKD organisms. The IFAT for BKD was originally described by Bullock and Stuckey (1975) and later modified by G. W. Camenisch (unpublished report) of the U.S. Fish and Wildlife Service (FWS), Eastern Fish Disease Laboratory. The complete procedure used in this study is described in our previous report (Novotny and Zaugg 1979). All dead and dying fish in the seawater pens were collected daily. Each fish was opened from the vent, external and internal lesions noted, and the procedures for culturing vibriosis and other gram negative bacteria (Novotny, Harrell, and Nyegaard 1975) were followed. The postmortems were classified as follows: 1. 2. 3. 4. 5. 6. 7. Negative (cause of death not determined). BKD (from lesions). Vibrio anguillarum--serotypes 775, 1669, or 7244. Vibrio sp. ERM (enteric redmouth). Furunculosis. Aeromonas hydrophilia (ex liquefaciens). - .- 8

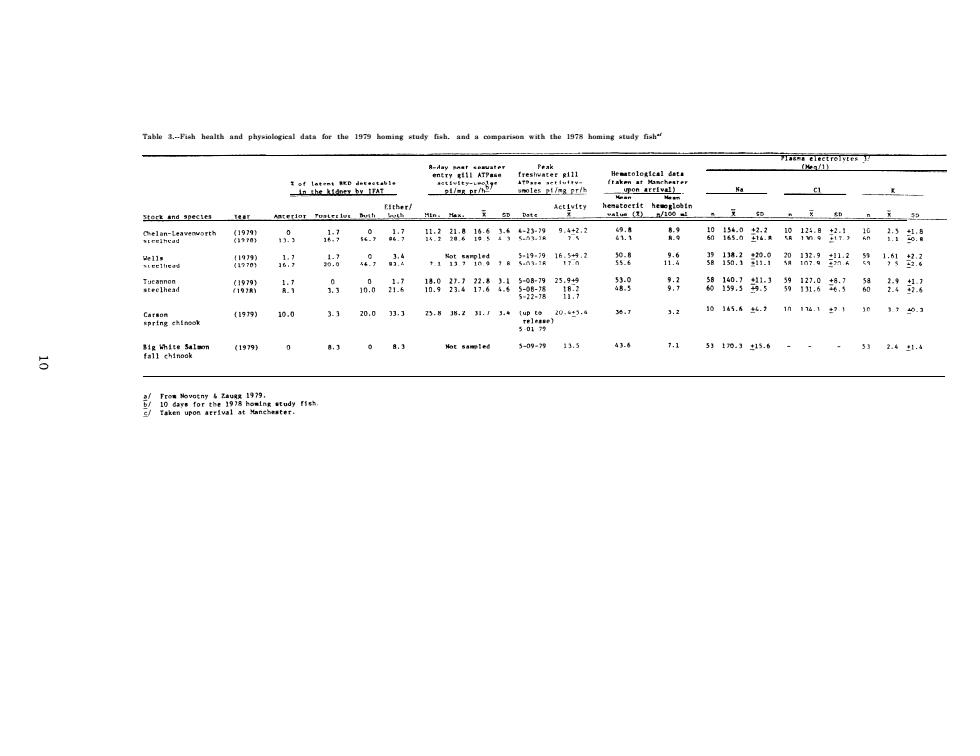
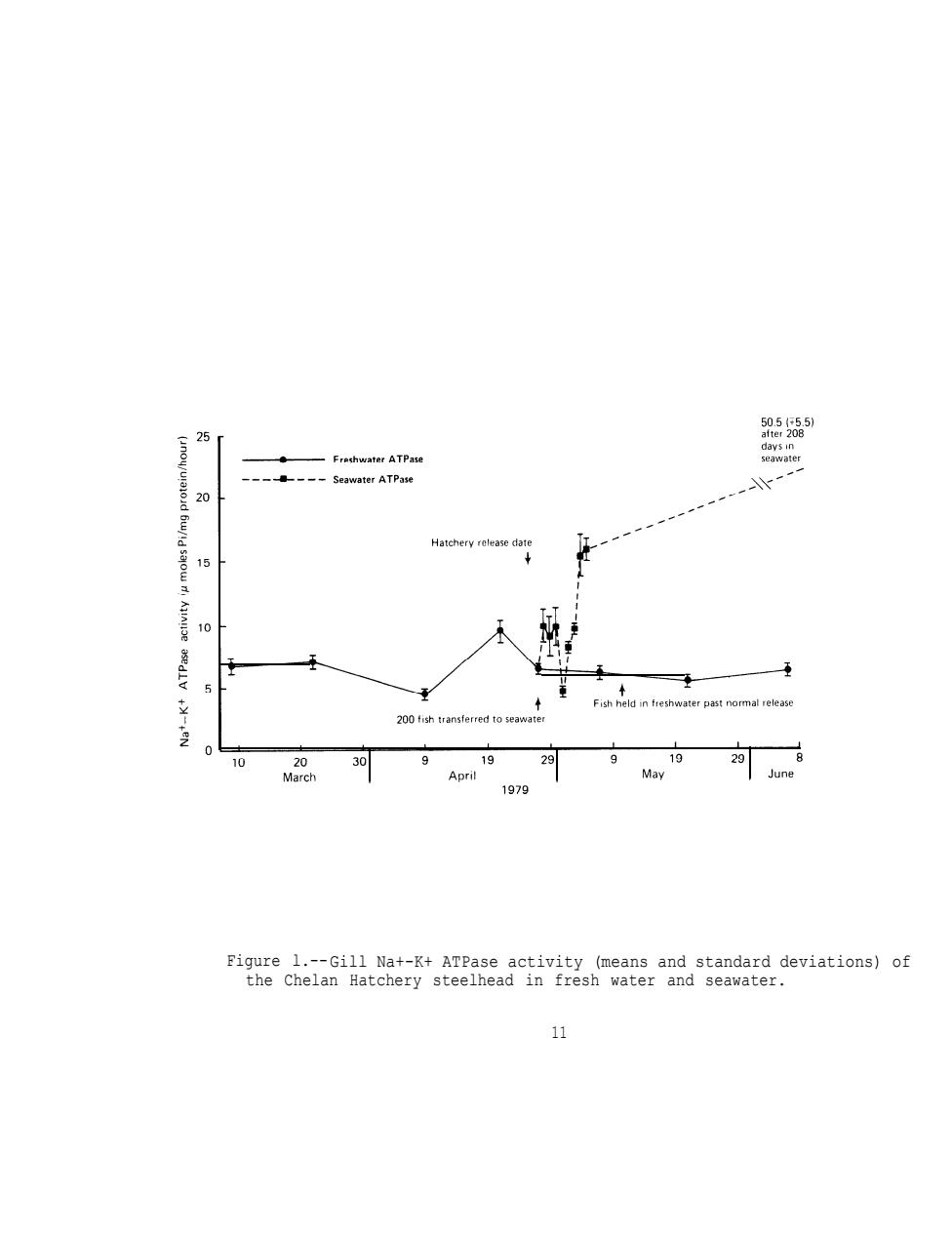
RESULTS AND DISCUSSION OF HATCHERY STEELHEAD SURVEYS Chelan Hatchery (Transferred to Leavenworth Hatchery)Steelhead Gill Na+-K+ATPase Since the phenomenon of elevation in gill sodium,potassium stimulated ATPase (Na+-K+ATPase)activity was first reported to be associated with Parr-smolt transformation in steelhead (Zaugg and Wagner 1973)and in in coho salmon,0.kisutch,(Zaugg and McLain 1970),numerous experiments have been conducted to verify these results and extend observations to other species.As a result,it has been conclusively shown that the rise in gill Na+-K+ATPase activity is one of the many physiological changes which occur at the time of Parr-smolt transformation. The average gill Na+-K+ATPase activity of Chelan Hatchery steelhead sampled in 1979 at Leavenworth Hatchery was not substantially different from 1978 (Table 3).Gill Na+-K+ATPase showed only a small rise in late April (Figure 1)with a peak mean value of 9.4.The absence of a greater increase in activity may have resulted from water temperatures which remained at the upper limit (13C)for good smoltification during late April and May (Zaugg et al.1972).The average fork length (20.8 cm) was similar to 1978 (21.0 cm),but the average weight (98.1 g)was up from 1978(79.4g). Plasma Electrolytes A compilation of data on rainbow trout by Miles and Smith (1968)and Hickman et al.(1964)suggests expected normal or near normal plasma electrolyte values in fresh water of 130 to 172 meq (milliequivalents)/1 for Nat,1.4 to 6.0 meq/l for K+,and 111 to 155 meq/l of cl-. Table 4 lists some known values for steelhead trout from available published literature,including data for the Dworshak Hatchery steelhead
RESULTS AND DISCUSSION OF HATCHERY STEELHEAD SURVEYS Chelan Hatchery (Transferred to Leavenworth Hatchery) Steelhead Gill Na+-K+ ATPase Since the phenomenon of elevation in gill sodium, potassium stimulated ATPase (Na+-K+ ATPase) activity was first reported to be associated with Parr-smolt transformation in steelhead (Zaugg and Wagner 1973) and in in coho salmon, 0. kisutch, (Zaugg and McLain 1970), numerous experiments - have been conducted to verify these results and extend observations to other species. As a result, it has been conclusively shown that the rise in gill Na+-K+ ATPase activity is one of the many physiological changes which occur at the time of Parr-smolt transformation. The average gill Na+-K+ ATPase activity of Chelan Hatchery steelhead sampled in 1979 at Leavenworth Hatchery was not substantially different from 1978 (Table 3). Gill Na+-K+ ATPase showed only a small rise in late April (Figure 1) with a peak mean value of 9.4. The absence of a greater increase in activity may have resulted from water temperatures which remained at the upper limit (13°C) for good smoltification during late April and May (Zaugg et al. 1972). The average fork length (20.8 cm) was similar to 1978 (21.0 cm), but the average weight (98.1 g) was up from 1978 (79.4 g). Plasma Electrolytes A compilation of data on rainbow trout by Miles and Smith (1968) and Hickman et al. (1964) suggests expected normal or near normal plasma electrolyte values in fresh water of 130 to 172 meq (milliequivalents)/l for Na+, 1.4 to 6.0 meq/l for K+, and 111 to 155 meq/l of Cl-. Table 4 lists some known values for steelhead trout from available published literature, including data for the Dworshak Hatchery steelhead 9
78 :超8.设:“日日 ”油日::8,州超 品品识批肚社器”游出 }88}88沿 m0.0 31009.3 1,g0145.44.1n1161313n0.3 5品4oe 8.3 4 ,1 24 彭二m
Table 3.--Fish health and physiological data for the 1979 homing study fish. and a comparison with the 1978 homing study fisha/
25 愿5 —Frethwater ATPase --------Seawater ATPase 20 15 10 Fish held reshwater past normal release 19 20 9 19 29 May June 1979 Fiqure 1.--Gill Na+-K+ATPase activity (means and standard deviations)of the Chelan Hatchery steelhead in fresh water and seawater. 11
Figure l.--Gill Na+-K+ ATPase activity (means and standard deviations) of the Chelan Hatchery steelhead in fresh water and seawater. 11