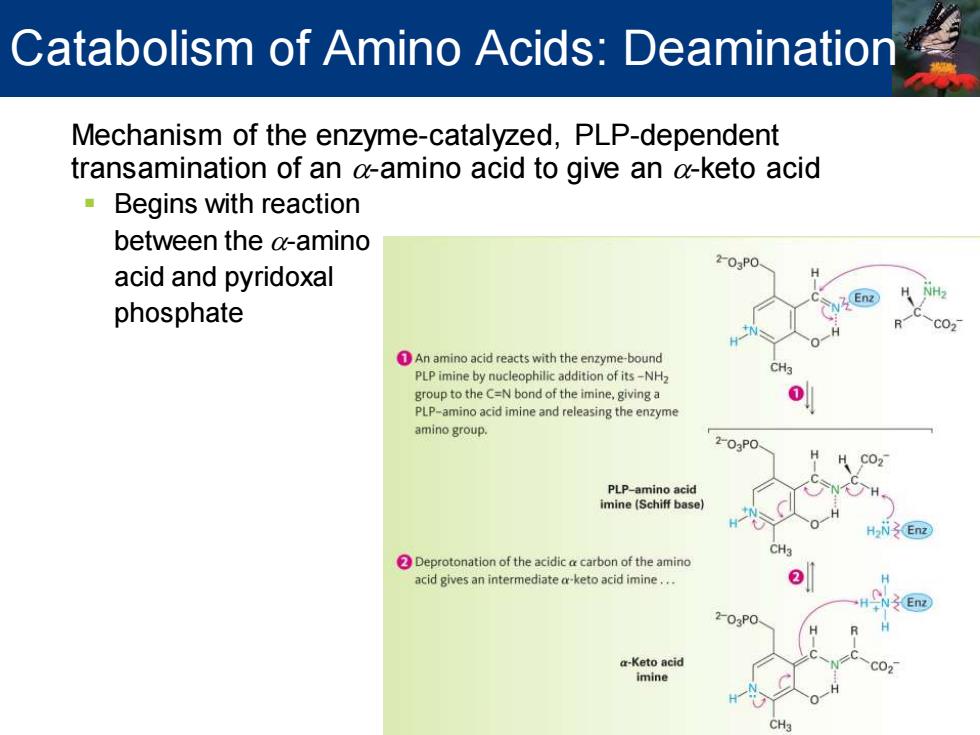
Catabolism of Amino Acids:Deamination Mechanism of the enzyme-catalyzed,PLP-dependent transamination of an a-amino acid to give an a-keto acid 。Begins with reaction between the a-amino 203P0 acid and pyridoxal En phosphate C02 DAn amino acid reacts with the enzyme-bound PLP imine by nucleophilic addition of its-NH2 group to the C=N bond of the imine,giving a PLP-amino acid imine and releasing the enzyme amino group. 203P0 PLP-amino acid imine(Schiff base】 HaN Enz Deprotonation of the acidic a carbon of the amino acid gives an intermediate a-keto acid imine... 0 -N3En 203P0 a-Keto acid imine
Mechanism of the enzyme-catalyzed, PLP-dependent transamination of an a-amino acid to give an a-keto acid ▪ Begins with reaction between the a-amino acid and pyridoxal phosphate Catabolism of Amino Acids: Deamination
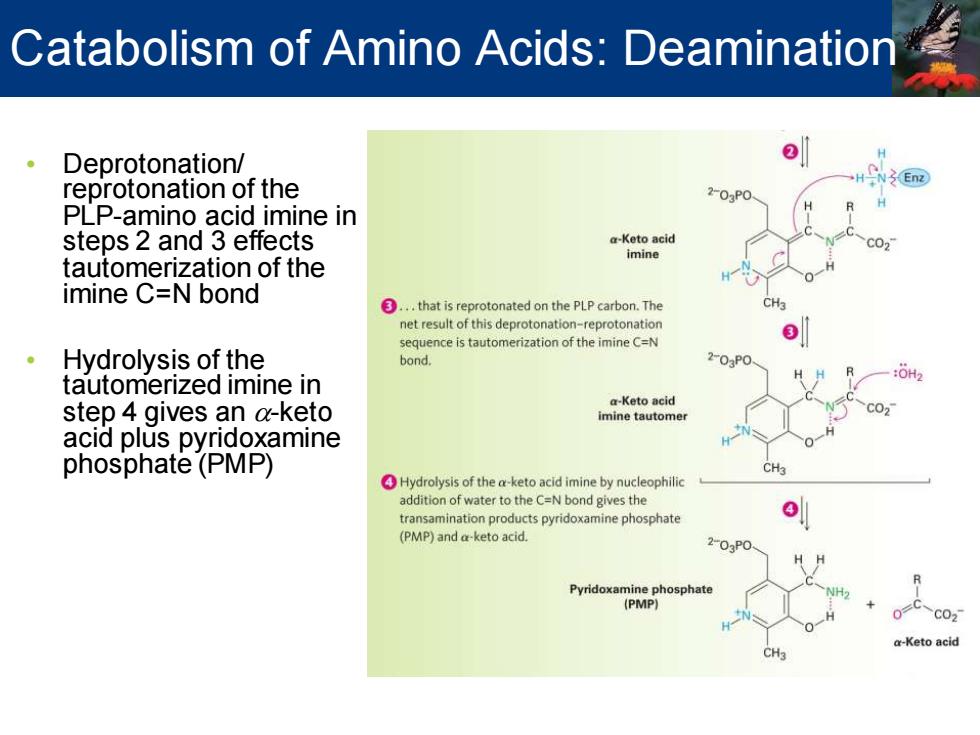
Catabolism of Amino Acids:Deamination Deprotonation/ reprotonation of the 2-03P0 PLP-amino acid imine in steps 2 and 3 effects a-Keto acid imine tautomerization of the imine C=N bond ③.,that is reprotonated on the PLP carbon.The net result of this deprotonation-reprotonation sequence is tautomerization of the imine C=N Hydrolysis of the bond. 203P0 HH tautomerized imine in step 4 gives an a-keto a-Keto acid imine tautomer acid plus pyridoxamine phosphate(PMP) Hydrolysis of the a-keto acid imine by nucleophilic addition of water to the C=N bond gives the transamination products pyridoxamine phosphate (PMP)and a-keto acid. 2-03P0 Pyridoxamine phosphate (PMP) C021 -Keto acid CH
• Deprotonation/ reprotonation of the PLP-amino acid imine in steps 2 and 3 effects tautomerization of the imine C=N bond • Hydrolysis of the tautomerized imine in step 4 gives an a-keto acid plus pyridoxamine phosphate (PMP) Catabolism of Amino Acids: Deamination