
Since two-body interactions are many times more common than three-body interactions;three-body interactions are many times more numerous than four-body interactions,etc.,the contributions to Z of the successively higher-ordered terms decrease rapidly
Since two Since two-body interactions are many times more body interactions are many times more common than three common than three-body interactions; three body interactions; three-body interactions are many times more numerous than four interactions are many times more numerous than four-body interactions, etc., the contributions to Z of the successively interactions, etc., the contributions to Z of the successively higher-ordered terms decrease rapidly. ordered terms decrease rapidly

3.3 The Ideal Gas 3.3.1 The equation of state of the ideal gas PV=RT PV =1 RT Where P -the gas of pressure V-the gas of molar volume T-temperature R-constant
3.3 The Ideal Gas 3.3 The Ideal Gas 3.3.1 The equation of state of the ideal gas Where P -the gas of pressure V -the gas of molar volume T -temperature R -constant PV RT Z PV RT = = = 1

3.3.2 Internal energy of the ideal gas: U=U(T) The Internal energy of the ideal gas is a function of temperature only
3.3.2 Internal energy of the ideal gas: U UT = ( ) The Internal energy of the ideal gas is a function of temperature only
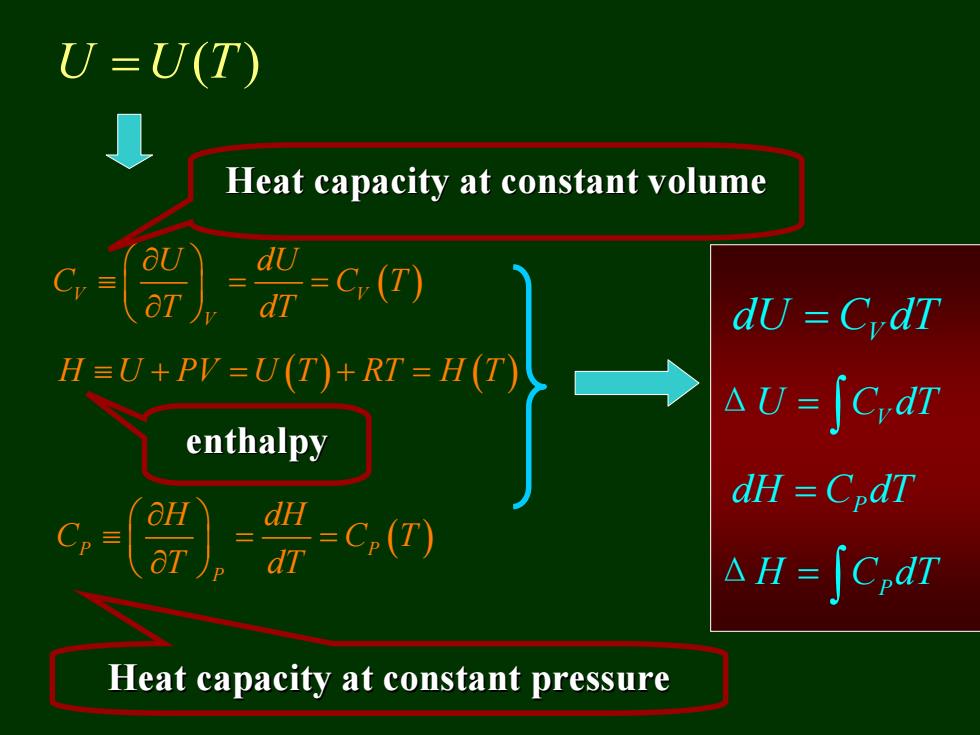
U=U(T) Heat capacity at constant volume dy CrdT HEU+PV=UT+RI=H(八 nu-SC,dt enthalpy dH =CpdT dH =C(T) dT △H=∫C,dn Heat capacity at constant pressure
U UT = ( ) V V ( ) V U dU C C T T dT ⎛ ⎞ ∂ ≡ == ⎜ ⎟ ⎝ ⎠ ∂ H U PV U T RT H T ≡+ = + = ( ) ( ) P P ( ) P H dH C C T T dT ⎛ ⎞ ∂ ≡ == ⎜ ⎟ ⎝ ⎠ ∂ V dU C dT = ΔU C dT = ∫ V P dH C dT = ΔH C dT = ∫ P Heat capacity at constant volume Heat capacity at constant volume enthalpy enthalpy Heat capacity at constant pressure Heat capacity at constant pressure

Equation for Process Calculations:Ideal Gases Main objects: calculating the work and heat for a process For any mechanically reversible closed-system process: according to The First Law do dw du 光c →dg=CrdT+PdW dw=-Pdv
Equation for Process Calculations: Ideal Gases Equation for Process Calculations: Ideal Gases For any mechanically reversible closed For any mechanically reversible closed-system process: process: dQ dW dU + = dW PdV = − V dQ C dT PdV = + Main objects: Main objects: calculating the work and heat for a process calculating the work and heat for a process according to The First Law according to The First Law V ( ) dU C T dT =