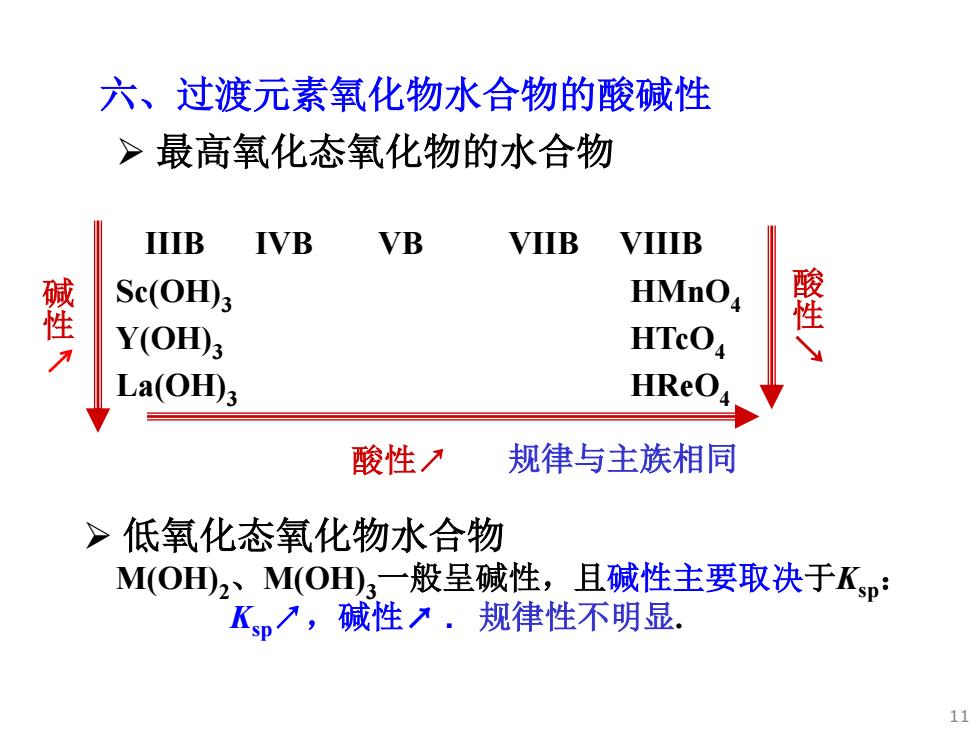
六、过渡元素氧化物水合物的酸碱性 >最高氧化态氧化物的水合物 IIIB IVB VB VIIB VIIIB 壁 Sc(OH田3 HMnO 璧 Y(OH); HTcO La(OH)3 HReO 酸性刀 规律与主族相同 >低氧化态氧化物水合物 M(OHD2、M(OH3一般呈碱性,且碱性主要取决于Ksp: Kp7,碱性入.规律性不明显. 11
六、过渡元素氧化物水合物的酸碱性 ¾ 低氧化态氧化物水合物 M(OH)2、M(OH)3一般呈碱性,且碱性主要取决于Ksp: Ksp↗,碱性↗ . 规律性不明显. ¾ 最高氧化态氧化物的水合物 碱 性 IIIB IVB VB VIIB VIIIB Sc(OH)3 HMnO4 Y(OH)3 HTcO4 La(OH)3 HReO4 酸性↗ 酸 性 ↗ 规律与主族相同 11

>同一元素,不同氧化态 按R-O-H模型,=Z*/r7,则R-O-H酸式电离倾向↑ V(OH)2 V(OH); V(OH) HVO: 弱B 更弱B AB A Cr(OH)2 Cr(OH)3 H,CrO B BA A Fe(OH) Fe(OH)3 B BA 同一元素低价态化合物碱性比高价态碱性强。 12
按R-O-H模型,I* = Z * / r ↗, 则R-O-H 酸式电离倾向↑ V(OH)2 V(OH)3 V(OH)4 HVO3 弱B 更弱B AB A Cr(OH)2 Cr(OH)3 H2CrO4 B BA A Fe(OH)2 Fe(OH)3 B BA 同一元素低价态化合物碱性比高价态碱性强。 ¾ 同一元素,不同氧化态 12

七、配合物: 过渡元素有很强的配位能力,因为有空的价轨道,可以与 配位原子形成σ配键;又有富的d电子,可与配体的π*反键轨 道或nd空轨道形成反馈π键→形成配合物倾向比主族M+大 得多: >①(n-1)d与s能量相近,n-1)d电子参与成键; >②d区M+:(9~17)e构型,(强极化力+大变形性)。 与配体互相极化,使M-L键共价性↑ 1.羰基配合物:通常金属氧化态较低(0,-1,+1): 2.羰基簇合物(分子中含有M一M键的化合物): 1)羰基簇合物中金属原子多为低氧化态并具有合适的d轨道。 2)金属一金属键(M一Ⅻ是原子簇合物最基本的共同特点。 3. 双氮配合物:N2与CO是等电子体,形成双氮配合物时,存在 双重键; 4. 乙烯配合物 13
¾① (n-1) d 与 ns 能量相近,(n-1)d电子参与成键; ¾② d 区 Mn+: (9 a 17)e构型, (强极化力 + 大变形性)。 与配体互相极化,使M-L键共价性↑ 七、配合物: 过渡元素有很强的配位能力,因为有空的价轨道,可以与 配位原子形成σ配键;又有富的d电子,可与配体的π *反键轨 道或nd空轨道形成反馈π键 形成配合物倾向 比主族Mn+大 得多: 1. 羰基配合物:通常金属氧化态较低(0, -1, +1); 2. 羰基簇合物 (分子中含有M—M键的化合物): 1) 羰基簇合物中金属原子多为低氧化态并具有合适的d轨道。 2) 金属-金属键(M-M) 是原子簇合物最基本的共同特点。 3. 双氮配合物: N2与CO是等电子体,形成双氮配合物时,存在 双重键; 4. 乙烯配合物 13
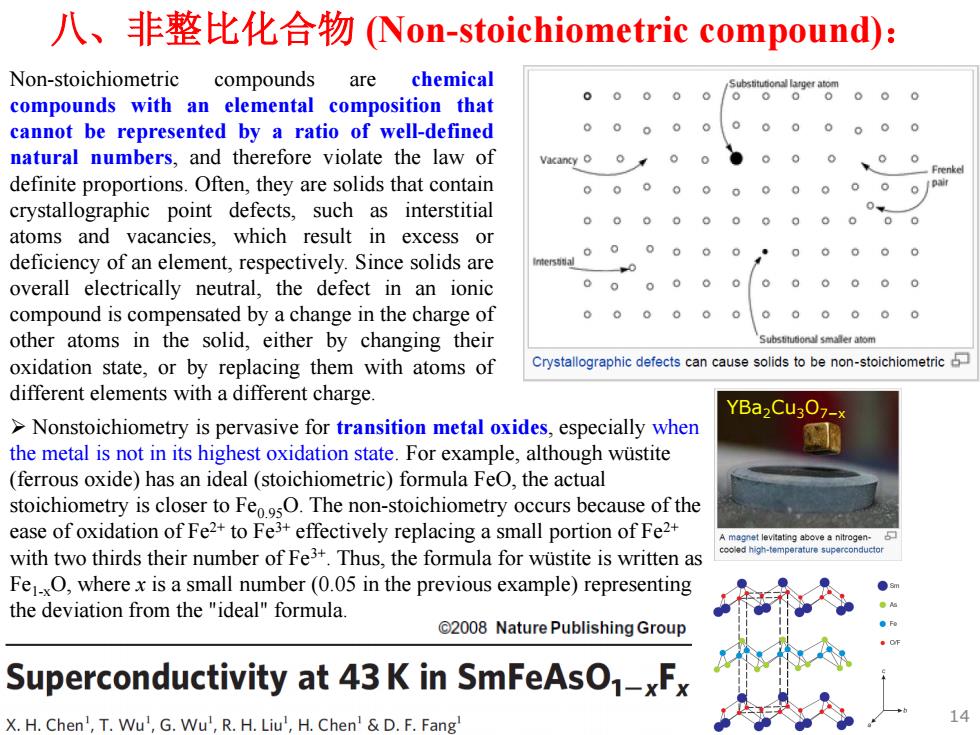
八、非整比化合物(Non-stoichiometric compound): Non-stoichiometric compounds are chemical compounds with an elemental composition that cannot be represented by a ratio of well-defined natural numbers,and therefore violate the law of definite proportions.Often,they are solids that contain 000.0000 0 crystallographic point defects,such as interstitial 0000000 0 。 atoms and vacancies,which result in excess or deficiency of an element,respectively.Since solids are 0 0000 0 0 overall electrically neutral,the defect in an ionic 00 00 0 compound is compensated by a change in the charge of other atoms in the solid,either by changing their oxidation state,or by replacing them with atoms of Crystallographic defects can cause solids to be non-stoichiometric different elements with a different charge. YBa2Cu302-× >Nonstoichiometry is pervasive for transition metal oxides,especially when the metal is not in its highest oxidation state.For example,although wustite (ferrous oxide)has an ideal(stoichiometric)formula FeO,the actual stoichiometry is closer to FeosO.The non-stoichiometry occurs because of the ease of oxidation of Fe2+to Fe3+effectively replacing a small portion of Fe2+ magnet levitating above a nitrogen with two thirds their number of Fe3+.Thus,the formula for wustite is written as cooea high-temperature superconducto Fe1O,where x is a small number(0.05 in the previous example)representing the deviation from the"ideal"formula. 2008 Nature Publishing Group Superconductivity at 43 K in SmFeAsO-xF X.H.Chen',T.Wu',G.Wu',R.H.Liu',H.Chen'&D.F.Fang
八、非整比化合物 (Non-stoichiometric compound): Non-stoichiometric compounds are chemical compounds with an elemental composition that cannot be represented by a ratio of well-defined natural numbers, and therefore violate the law of definite proportions. Often, they are solids that contain crystallographic point defects, such as interstitial atoms and vacancies, which result in excess or deficiency of an element, respectively. Since solids are overall electrically neutral, the defect in an ionic compound is compensated by a change in the charge of other atoms in the solid, either by changing their oxidation state, or by replacing them with atoms of different elements with a different charge. ¾ Nonstoichiometry is pervasive for transition metal oxides, especially when the metal is not in its highest oxidation state. For example, although wüstite (ferrous oxide) has an ideal (stoichiometric) formula FeO, the actual stoichiometry is closer to Fe0.95O. The non-stoichiometry occurs because of the ease of oxidation of Fe2+ to Fe3+ effectively replacing a small portion of Fe2+ with two thirds their number of Fe3+. Thus, the formula for wüstite is written as Fe1-xO, where x is a small number (0.05 in the previous example) representing the deviation from the "ideal" formula. YBa2Cu3O7−x 14

3B 4B 5B 6B 7B 8B 11B 12B 22 23 24 25 6 27 28 9 30 Sc Cr Mn Fe Co i Cu Zn [Ar)4s23d 4s23d3 [Ar)4s'3d5 [Ar]4s23d6 [Ar]4s23d [Ar23d10 scandium tandum chromum iron cobalt 44.96 47.88 50.94 52.00 54,94 55.85 58.93 58.69 63.55 65.39 0 41 42 43 44 45 46 47 8 Y Zr Nb Mo Ru Rh Pd Ag Cd a k410 w15s4d10 K5s24d10 palladium silver cadmium 88.91 91.22 92.91 95.94 (98) 101.1 102.9 106.4 107.9 112.4 57 72 74 75 76 77 78 79 80 a米 Hf Ta W Re Os Ir Pt Au Hg 624f145d Xe]6s 4f145d e16s244d 90.2 platinum 138.9 183.9 186.2 190.2 195.1 197.0 2803 89 104 105 106 107 108 109 10 11 112 Rf Db Sg Bh Hs Mt Ds dubnium bohrum hassium meitnerium armstadtuin (227 257 260 263) 262) (265) (266) (271D (272) 277 15
15